Method of extracting active molecules from natural resins and use thereof
A technology of active molecules, extracts, applied in the field of preparation of food compositions or supplements or pharmaceutical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1 - Myrrh
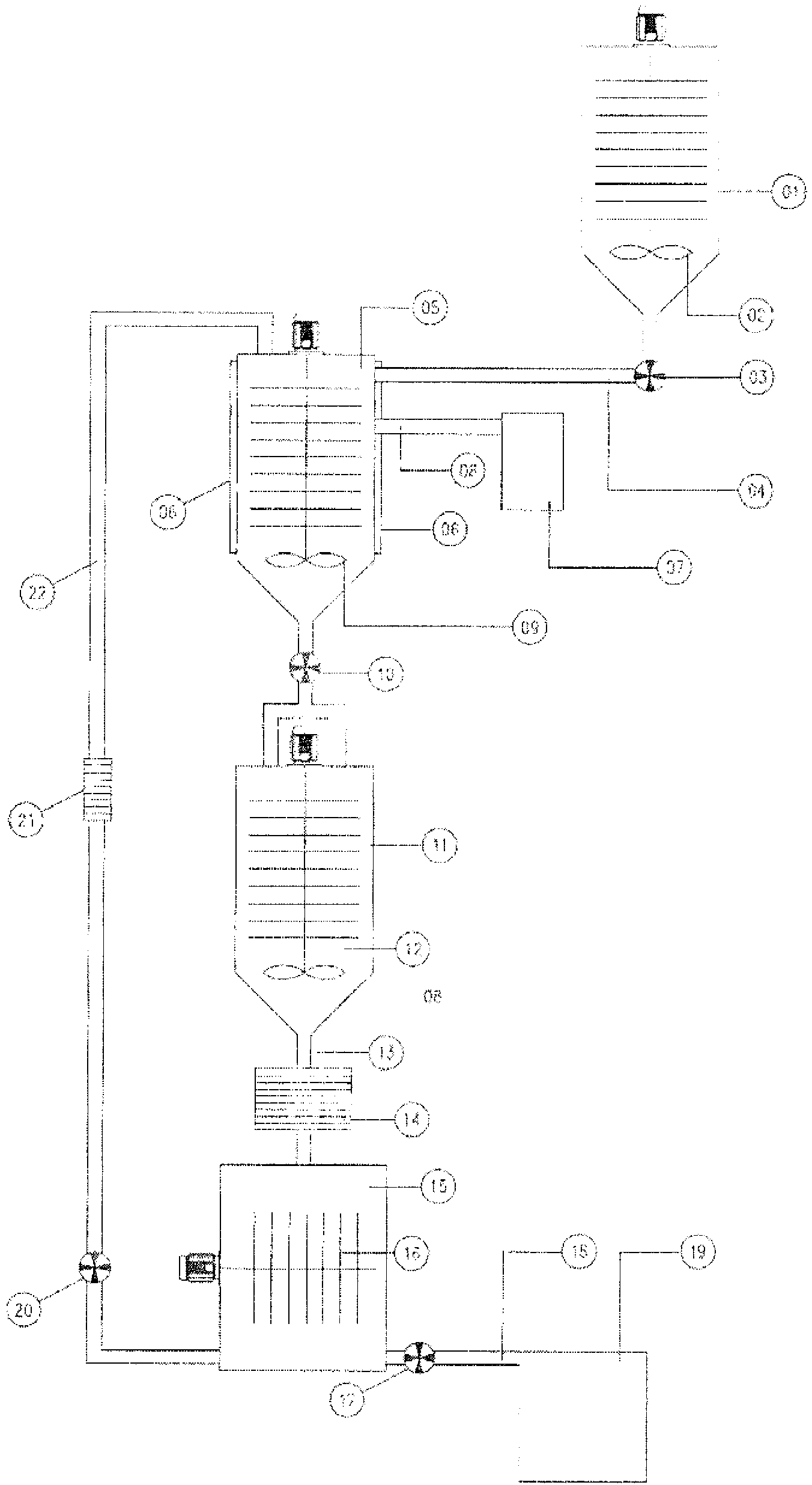
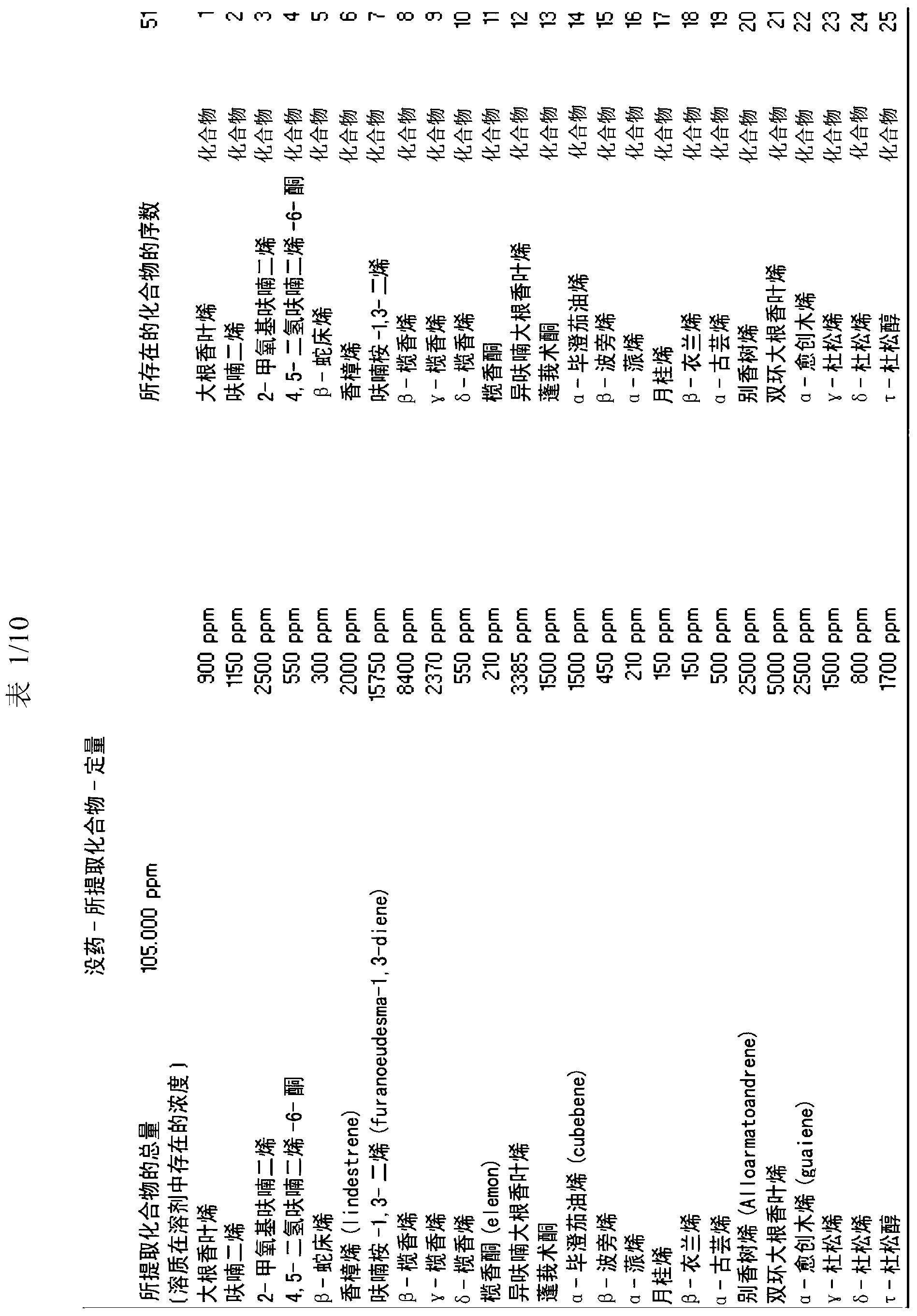
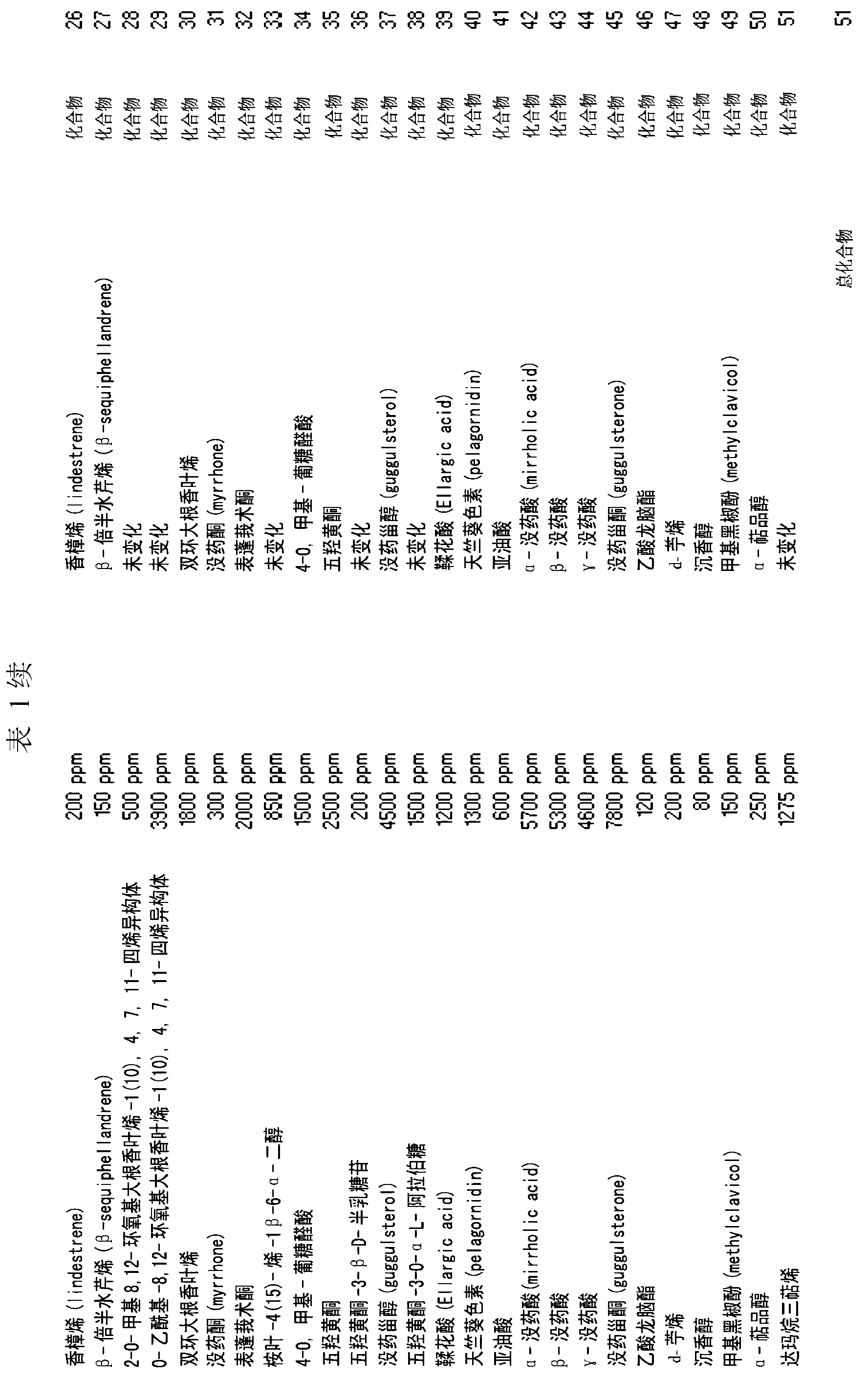
[0147] The extraction method of the present invention is used to extract active molecules contained in myrrh. The extraction apparatus was loaded with 1500 ml of extraction solvent comprising: acetic acid (12% in water), 58% by weight, and distilled water, 42% by weight. The solvent:myrrh ratio was 15:1 by weight; 100 g of myrrh was finely ground (particle size inclusive 100-120 microns). Carbon dioxide was used in an amount of 30 ml (2% by volume, relative to the total volume of the extraction solvent). A predetermined amount of extraction solvent (1500 ml) was introduced into tank 01 and kept under stirring at 50 rpm using stirring device 02 for 15 minutes. The extraction solvent flows from tank 01 to tank 05 using pump 03 and connection 04 . Tank 05 is equipped with heating means 06 and stirring means 09 . 100 g of myrrh were weighed and ground (particle size comprised 40-100 microns), introduced into tank 11 and kept under stirring using stir...
Embodiment 2
[0148] Example 2 - Agarwood
[0149] The extraction method of the present invention is used to extract active molecules contained in agarwood. Example 2 was carried out with the same procedure as described in Example 1, with the only differences as described below. The extraction apparatus was loaded with 1500 ml of extraction solvent comprising: acetic acid (12% in water), 58% by weight; 99% ethanol, 20% by weight, and distilled water, 22% by weight. The solvent: agarwood ratio was 15:1 by weight; 100 g of agarwood was finely ground (particle size inclusive 100-120 microns). Carbon dioxide was used in an amount equal to 30 ml (2% by volume, relative to the total volume of the extraction solvent). As can be seen from Table 2, 46 active molecules were extracted from Agarwood. The extraction yield by weight was 125,000 ppm, corresponding to 12.5% of 100 g of agarwood, relative to the total weight of extractable substances initially present in 100 g of agarwood.
Embodiment 3
[0150] Example 3 - Brachyphyllum
[0151]The extraction method of the present invention was used to extract active molecules contained in Pittonia brevis (abbreviated as TP). Example 3 was carried out with the same operating steps as described in Example 1, with the only differences as described below. The extraction apparatus was loaded with 1500 ml of extraction solvent comprising: acetic acid (12% in water), 40% by weight, and distilled water, 60% by weight. Solvent:TP ratio was 15:1 by weight; 100 g of TP was finely ground (particle size inclusive 100-120 microns). Carbon dioxide was used in an amount equal to 45 ml (3% by volume, relative to the total volume of the extraction solvent). As can be seen from Table 3, 49 active molecules were extracted from Agarwood. The extraction yield by weight was 85,000 ppm, corresponding to 8.5% of 100 g of TP, relative to the total weight of extractable material initially present in 100 g of TP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com