Method for increasing solubility of 10-hydroxycamptothecin, product prepared by the method and applications of the product
A technology of hydroxycamptothecin and alkali solubility, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc. It can reduce the time and frequency of clinical administration, shorten the course of treatment and the method is simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
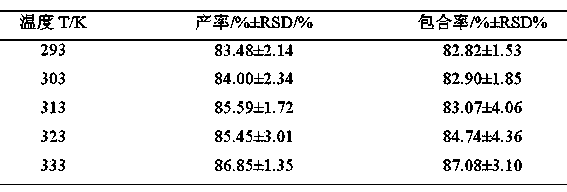
[0018] Example 1: Screening of 10-hydroxycamptothecin-seven-membered cucurbit inclusion complex process conditions
[0019] (1) Determine the optimal feed ratio (also known as the stoichiometric ratio) between the seven-membered cucurbitan and 10-hydroxycamptothecin
[0020] In four 100mL round-bottomed flasks, add about 0.5g seven-membered cucurbitan ring respectively, then add a certain amount of 10-hydroxycamptothecin respectively, so that the metering ratio (n Q[7] :n HCPT ) were 1:1, 2:1, 3:1, 4:1, respectively, and finally about 85mL of hydrochloric acid solution with a pH of 2.0 was added and fully stirred for 4 hours at a constant temperature of 40~45°C. After the reaction was completed, the solution in the round bottom flask was filtered while hot to remove undissolved precipitates to obtain a clear and translucent yellow-green filtrate. Then the filtrate was concentrated and dried at 40-45°C, scraped out and weighed, and the yield and inclusion rate were calcula...
Embodiment 2
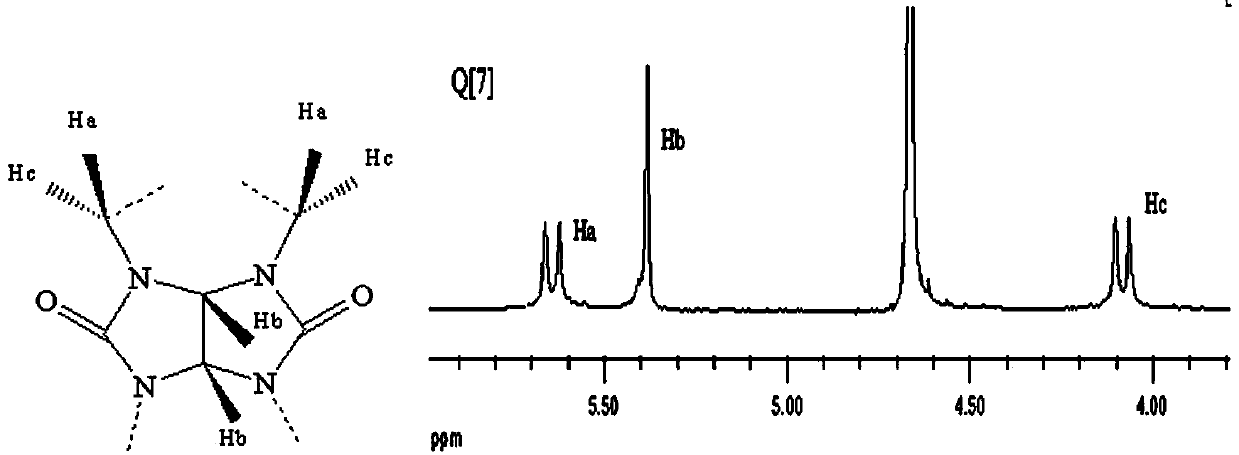
[0032] Example 2: Preparation of 10-hydroxycamptothecin-seven-membered cucurbit ring solid inclusion complex by co-evaporation
[0033] Weigh a certain amount of seven-membered cucurbit ring and hydroxycamptothecin (the stoichiometric ratio n Q[7] / n [HCPT] =3:1 Put it into a 100mL round bottom flask, add about 85mL of hydrochloric acid solution with a pH of 2.0, and stir fully at a constant temperature of 40~45°C for 4h. After the reaction was completed, the solution in the round bottom flask was filtered while hot to remove undissolved precipitates to obtain a clear and translucent yellow-green filtrate. Then the filtrate was evaporated to dryness by rotary evaporation, dried at 40-45°C, scraped off and weighed to obtain the seven-membered cucurbitanyl-hydroxycamptothecin solid inclusion compound.
Embodiment 3
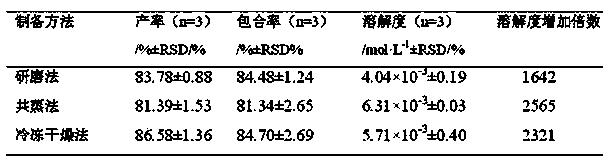
[0034] Example 3: Preparation of 10-hydroxycamptothecin-seven-membered cucurbit ring solid inclusion compound by grinding method
[0035] Weigh the stoichiometric ratio (n Q[7] :n HCPT ) with a ratio of 3:1 of Q[7] and HCPT solids were placed in an agate mortar, added about 1 mL of hydrochloric acid solution with a pH of 2.0, ground for 1 hour, and then dried in an oven at 40-45 °C to obtain .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com