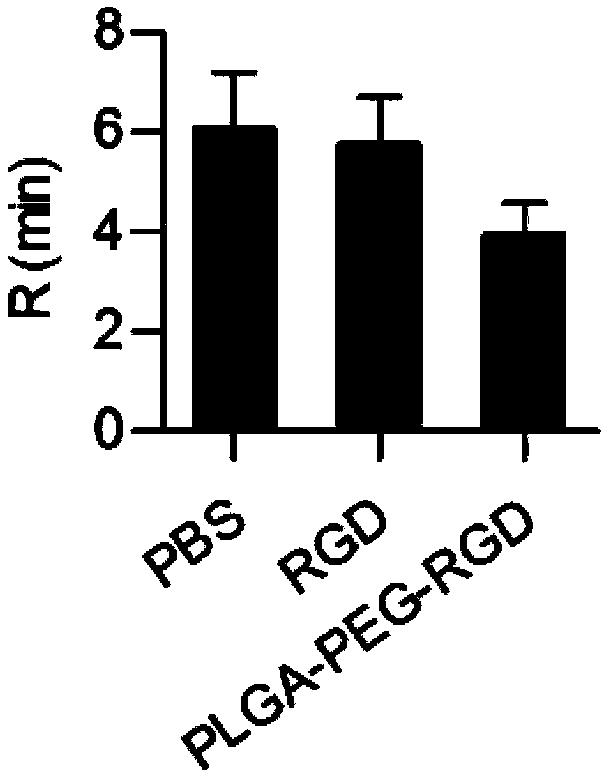
Application of artificial platelet PLAG-PEG-RCD to preparing systemic nanometer styptic for veins
A technology of PLGA-PEG-RGD and PLGA-PEG-COOH, which is applied in the field of artificial platelets, can solve problems such as the complexity of artificial platelet synthesis methods, and achieve the effects of improving self-rescue and mutual rescue capabilities, stable performance, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The method for preparing artificial platelet PLGA-PEG-RGD provided by the present invention may comprise the following steps:
[0058] Step 1: Synthesis of PLGA-NHS
[0059] According to the following chemical equation, synthesize the activated ester PLGA-NHS from carboxyl-terminated PLGA (Resomer503H) and NHS (N-hydroxysuccinimide);
[0060]
[0061] The meanings of x and y are the same as those defined above.
[0062] Step 2: Synthesis of PLGA-PEG block copolymer PLGA-PEG-COOH
[0063] The activated ester PLGA-NHS and polyethylene glycol (NH 2 -PEG-COOH) The block copolymer PLGA-PEG-COOH of the carboxyl-terminated PLGA-PEG connected by an amide bond;
[0064]
[0065] n has the same meaning as defined above.
[0066] Step 3: Synthesis of PLGA-PEG-NHS
[0067] According to the following chemical equation, the terminal carboxyl group of PLGA-PEG block copolymer PLGA-PEG-COOH is reacted with NHS under the action of EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodi...
Embodiment 1
[0082] Example 1. Preparation of artificial platelet PLGA-PEG-RGD loaded with LGA-PEG tripeptide RGD
[0083] This example describes in detail the process of preparing LGA-PEG-loaded RGD tripeptide artificial platelet PLGA-PEG-RGD:
[0084] Step 1: Synthesis of PLGA-NHS
[0085]According to the following chemical equation, the carboxyl-terminated PLGA (Resomer503H) and NHS are synthesized into the activated ester PLGA-NHS. The specific synthesis method is: under the protection of nitrogen, dissolve 3 g of PLGA (Resomer503H, x=272, y=272) in 10 mL of Anhydrous dichloromethane, then add EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide) 76μL (4.3equiv) and NHS (N-hydroxysuccinimide) 46mg ( 4equiv), stirred at room temperature for 6 hours, concentrated the reaction solution to 2-3mL, added diethyl ether to settle, stood still, and filtered to obtain a white crude product. solid;
[0086]
[0087] Step 2: Synthesis of PLGA-PEG block copolymer PLGA-PEG-COOH
[0088] The act...
Embodiment 2
[0102] The characterization of embodiment 2, PLGA-PEG-RGD nanoparticles
[0103] Suspend the nanoparticles obtained in Example 1 in a PBS buffer solution (5-25 mg / mL, preferably 20 mg / mL), and disperse them ultrasonically (power 250 watts) to obtain a new nanosphere solution for detection.
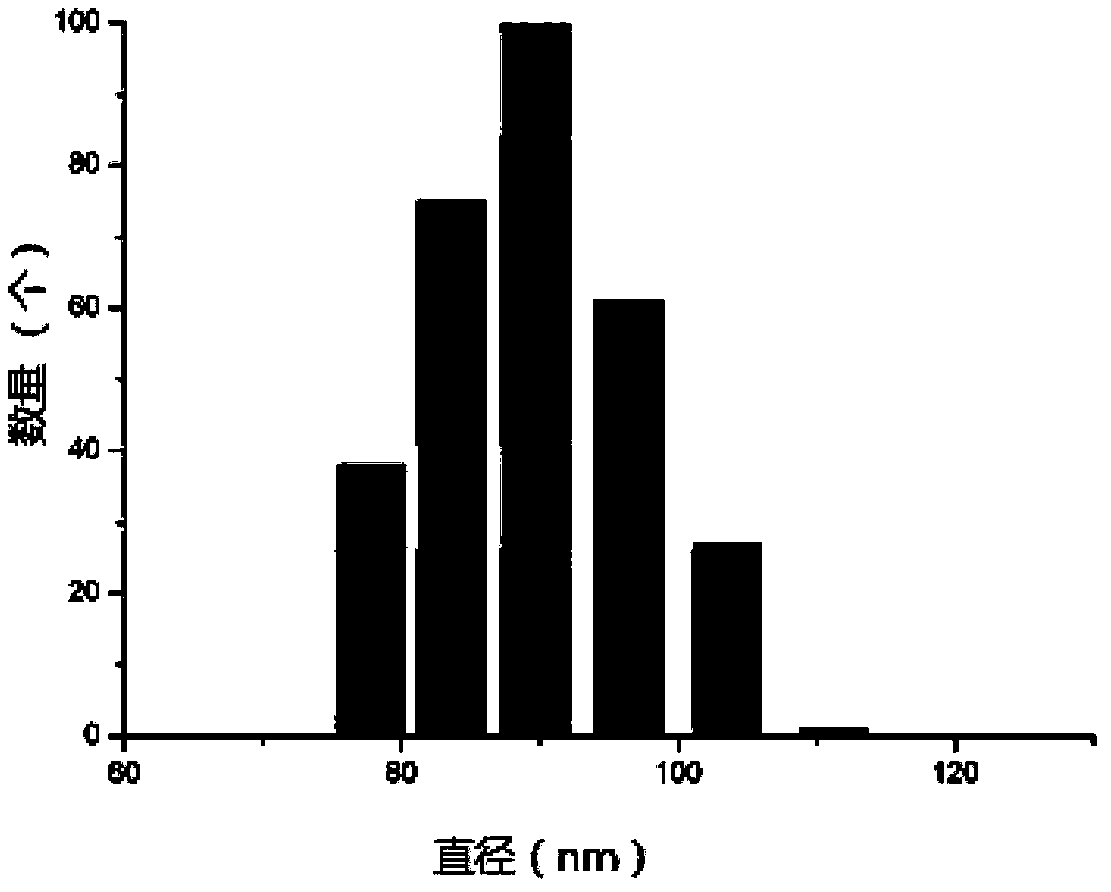
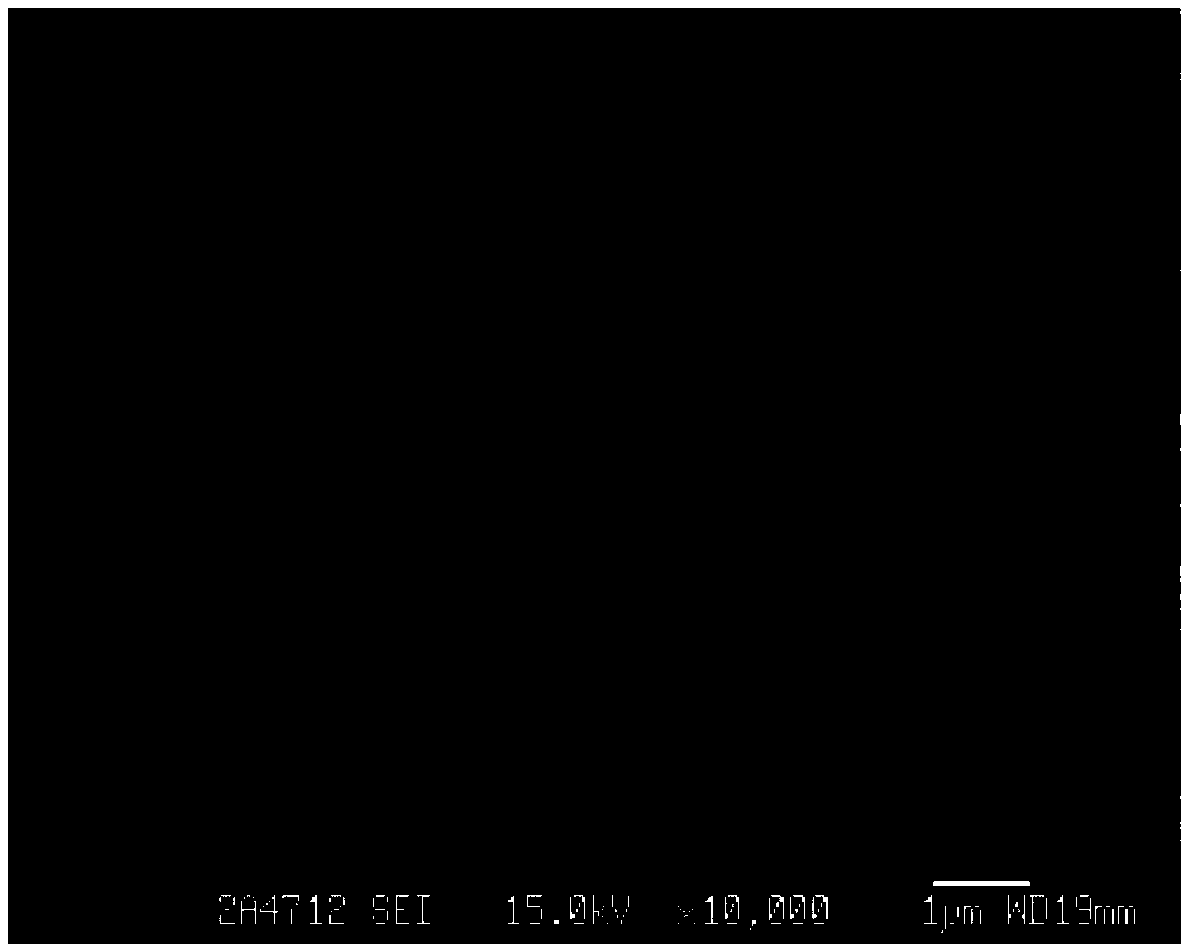
[0104] The effective diameter of the PLGA-PEG-RGD nanoparticles was measured by dynamic light scattering, and the morphology of the nanoparticles was observed by a scanning electron microscope. When acetonitrile is used, nano-scale particles are obtained under the action of ultrasound in a sonicator. The test results of dynamic light scattering (DLS) are as follows: figure 1 (Abscissa represents the diameter of the PLGA-PEG-RGD nanoparticle that obtains, and ordinate represents the quantity that detects) shown, effective particle diameter is 91.3nm, confirms that the size of PLGA-PEG-RGD nanoparticle is nanoscale; PLGA- The scanning electron micrographs of PEG-RGD nanoparticles are as fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com