Method for enriching lithium in carbonate type salt lake brine
A salt lake brine, carbonate type technology, applied in the directions of alkali metal sulfite/sulfite, chemical instruments and methods, lithium compounds, etc., to achieve the effect of low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
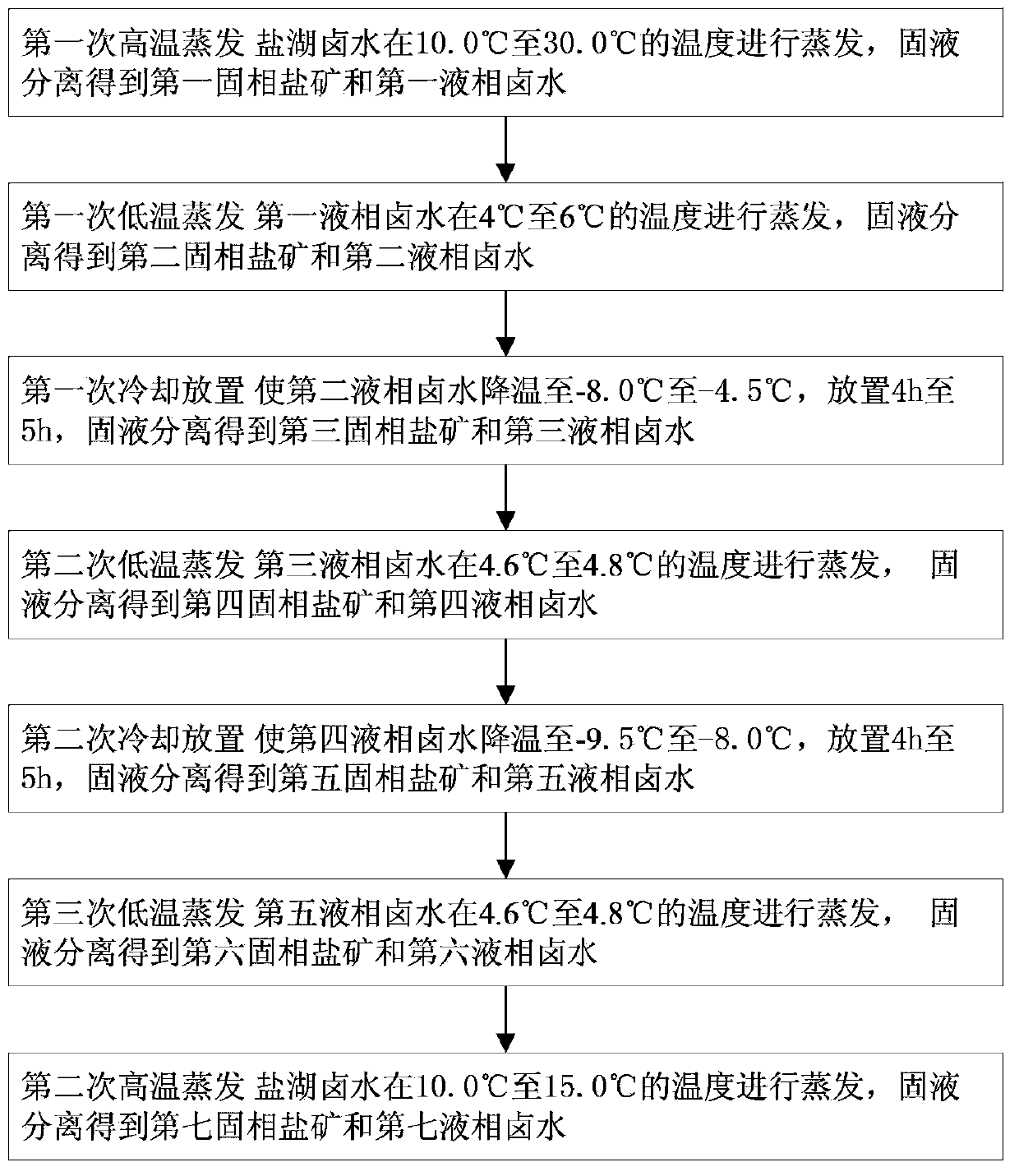
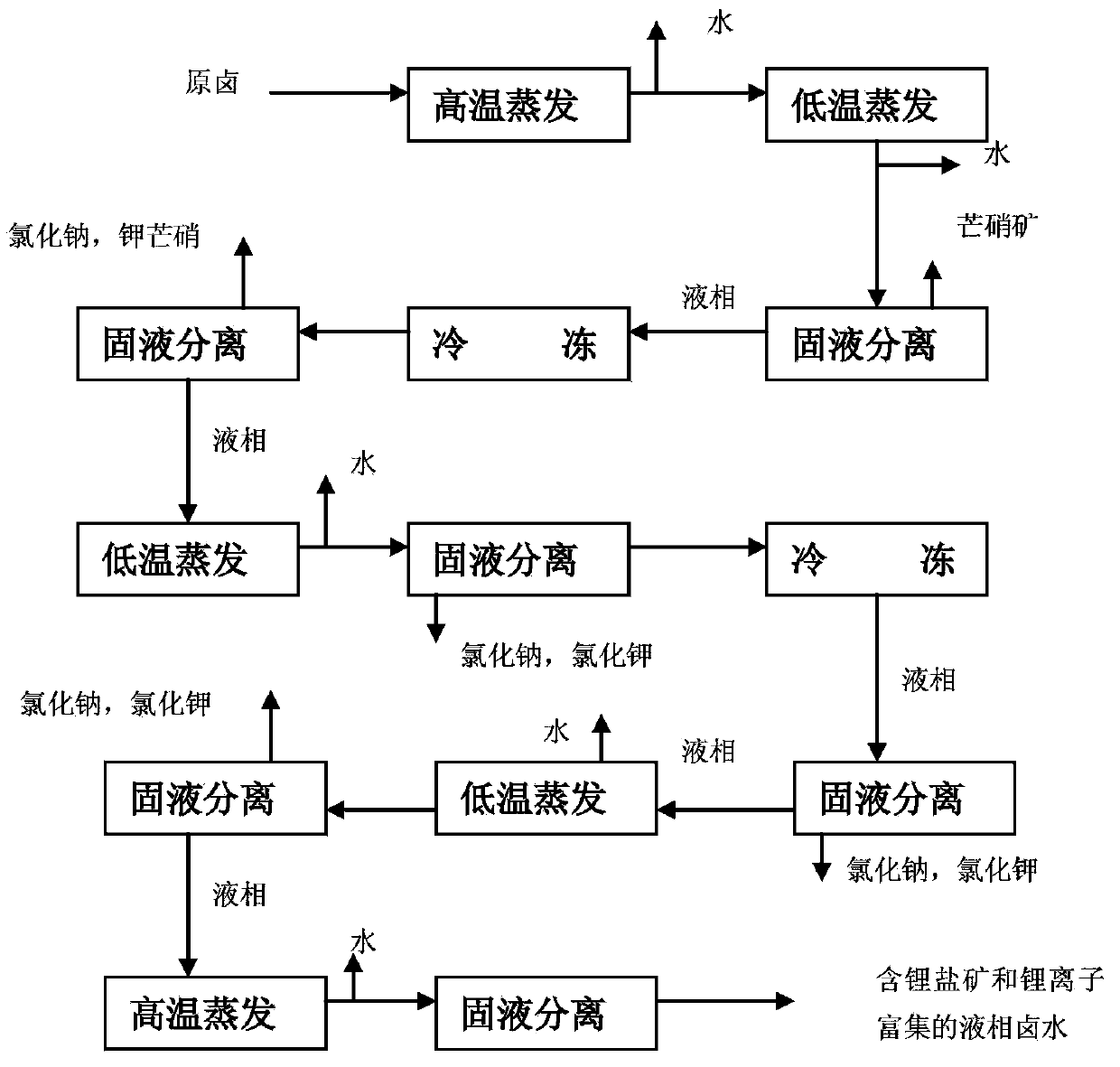
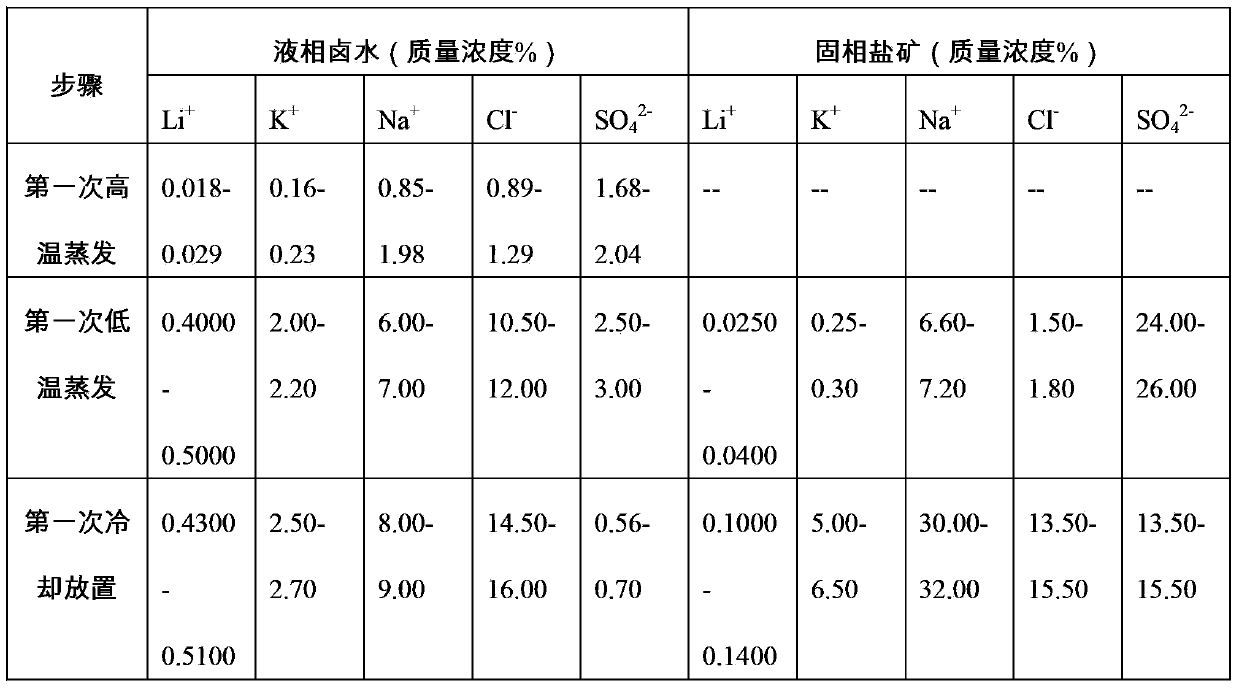
[0049] S101 The first high-temperature evaporation: After mixing the brine of a salt lake in Tibet evenly, place it in an evaporation pool for high-temperature evaporation (under the condition that the temperature of the brine in the salt lake is about 25°C), and obtain the first liquid-phase brine.
[0050] S102 The first low-temperature evaporation: Take 400kg of the first liquid-phase brine and place it in the environmental simulation room. Adjust the temperature of the simulation room to control the temperature of the brine at 4.0°C. The electric fan is intermittently exhausted at a wind speed of 2.80m / s. Monitoring Li in brine during evaporation + Concentration, when Li + When the mass concentration is 0.4000%, the brine and the evaporated salt mine are subjected to solid-liquid separation to obtain 26 kg of the second solid phase salt mine in the solid phase and 13.58 kg of the second liquid phase brine.
[0051] S103 Cooling for the first time: place the second liquid-...
Embodiment 2
[0061] S201 The first high-temperature evaporation: After mixing the brine of a salt lake in Tibet evenly, place it in an evaporation pool for high-temperature evaporation (under the condition that the temperature of the brine in the salt lake is about 10°C), and obtain the first liquid-phase brine.
[0062] S202 The first low-temperature evaporation: Take 400kg of the first liquid-phase brine and place it in the environmental simulation room. Adjust the temperature of the simulation room to control the temperature of the brine at 5.5°C. The electric fan is intermittently exhausted, and the wind speed is controlled at 3.50m / s. Monitoring Li in brine during evaporation + Concentration, when Li + When the mass concentration is 0.4500%, the brine and the evaporated salt mine are subjected to solid-liquid separation to obtain 30.0 kg of the second solid phase salt mine in the solid phase and 13.28 kg of the second liquid phase brine.
[0063] S203 Cooling for the first time: plac...
Embodiment 3
[0073] S301 The first high-temperature evaporation: After mixing the brine of a salt lake in Tibet evenly, place it in an evaporation pool for high-temperature evaporation (under the condition that the temperature of the brine in the salt lake is about 30°C), and obtain the first liquid-phase brine.
[0074] S302 The first low-temperature evaporation: Take 400kg of the first liquid-phase brine and place it in the environmental simulation room. Adjust the temperature of the simulation room to control the temperature of the brine at 6.0°C. The electric fan is intermittently exhausted, and the wind speed is controlled at 3.80m / s. Monitoring Li in brine during evaporation + Concentration, when Li + When the mass concentration is 0.5000%, the brine and the evaporated salt ore are subjected to solid-liquid separation to obtain 27.5kg of second solid-phase salt ore and 13.4kg of second liquid-phase brine.
[0075] S303 Cooling for the first time: place the second liquid-phase brine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com