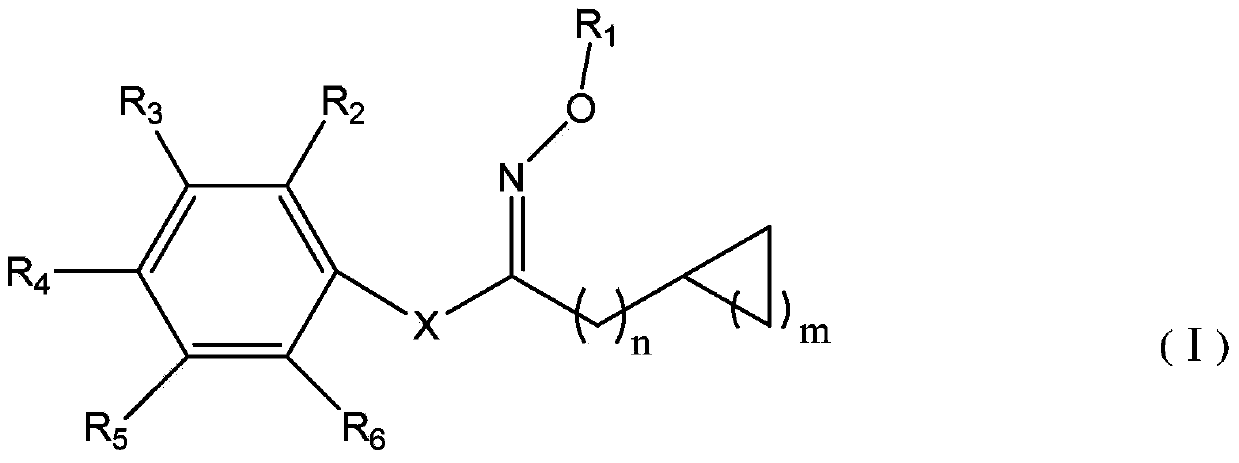
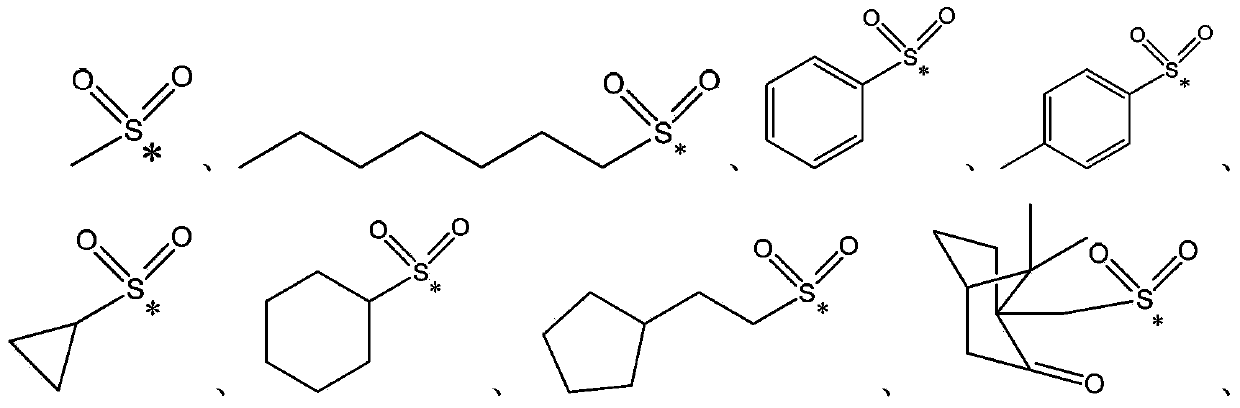
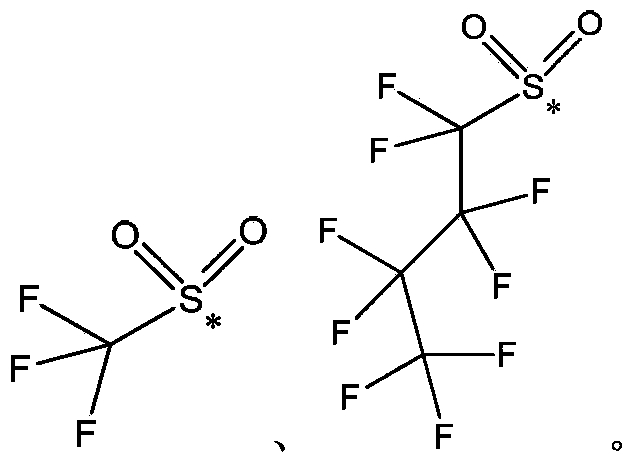
Oxime ester sulfonate type photo-acid generator
A photoacid generator and oxime sulfonate technology, which is applied in the field of oxime sulfonate photoacid generators, can solve the problems that affect popularization and application and market expectations, low photosensitive activity, and can not meet the exposure requirements, etc., and achieve excellent application performance , High photosensitivity, high photosensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of 1-[6-(2-thienyl)-9-ethylcarbazol-3-yl]-3-cyclopentyl-propane-1,2-dione-2-oxime methylsulfonate (i.e. Compound No.10)
[0029]
[0030] Step (1): Preparation of 3-(3-cyclopentylpropionyl)-6-(2-thienoyl)-9-ethylcarbazole
[0031]
[0032] Drop into 30g 9-ethylcarbazole, 21.6gAlCl in the 500ml four-necked flask 3 (Grinding), 150ml of dichloromethane, stirred, protected by argon, cooled in an ice bath, when the temperature dropped to 0°C, began to drop the mixture of 23.2g of 2-thiophenoyl chloride and 21g of dichloromethane, temperature controlled Below 10°C, add about 1.5h, continue to stir for 2h, then add 21.6g AlCl to the flask 3 (Grind finely), add dropwise a mixture of 27.2g cyclopentylpropionyl chloride and 20g dichloromethane, control the temperature below 10°C, drop it in about 1.5h, then raise the temperature to 15°C, continue stirring for 2h, and stop the reaction. Pour the reaction solution into dilute hydrochloric acid made of 400g ice ...
Embodiment 2
[0043] Preparation of 1-(6-o-methylbenzoyl-9-ethylcarbazol-3-yl)-3-cyclobutyl-propane-1-one oxime p-toluenesulfonate (Compound No. 2)
[0044]
[0045] Step (1): Preparation of 3-(3-cyclobutylpropionyl)-6-o-methylbenzoyl-9-ethylcarbazole
[0046]
[0047] Drop into 19.5g 9-ethylcarbazole, 13.5gAlCl in the 500ml four-necked flask 3 (Grind finely), 150ml of dichloromethane, stir, pass through argon protection, and cool in an ice bath. When the temperature drops to 0°C, start to drop a mixture of 15.5g of o-toluyl chloride and 15g of dichloromethane. The temperature was controlled below 10°C, the addition was completed in about 1.5 hours, and the stirring was continued for 2 hours, then 13.5g of AlCl was added to the flask 3 (Grind finely), add dropwise a mixture of 15g cyclobutylpropionyl chloride and 20g dichloromethane, keep the temperature below 10°C, drop it in about 1.5h, then raise the temperature to 15°C, continue to stir for 2h, stop the reaction. Pour the react...
Embodiment 3-4
[0056] Referring to the method shown in Example 2, the compounds of Examples 3 and 4 were prepared from the corresponding reagents. target compounds and their 1 H-NMR data are listed in Table 1.
[0057] Table 1
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com