Methods for treating metabolic disorders and obesity with GIP and GLP-1 receptor-active glucagon-based peptides
A GLP-1, therapeutic agent technology, applied in metabolic diseases, organic active ingredients, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0170] In an exemplary embodiment, the half-life extended GLP-1 / GIP co-agonist peptide of the present disclosure is an analog of native glucagon comprising (i) an amino acid comprising an imidazole side chain at position 1, (ii) A DPP-IV protected amino acid at position 2, (iii) optionally an acylated amino acid or an alkylated amino acid at any of positions 9, 10, 12, 16, 20 or 37-43, wherein an acyl or an alkyl group is optionally (iv) an alpha-helix stabilizing amino acid at one or more of positions 16-21, and (v) up to ten (e.g., up to 2, 3, 4, 5, 6, 7, 8, 9 or 10) other amino acid modifications that retain the desired GIP and GLP-1 activity.
[0171] In any of the exemplary embodiments of this section, the amino acid at position 1 may be His or a derivative of His. In any of the exemplary embodiments, the amino acid at position 2 is an α,α-disubstituted amino acid. In any exemplary embodiment, the α,α-disubstituted amino acid comprises R1 and R2 (each of which is bonded...
Embodiment 1
[0174] Example 1: Half-life extended GLP-1 / GIP co-agonist peptides with an extended half-life of about 5 days are well tolerated
[0175] A phase I, randomized, placebo-controlled, continuous, single-ascending, two-period study in healthy male subjects to assess the safety, tolerability, pharmacokinetics of GLP-1 / GIP co-agonist peptides with extended half-life ( PK) and pharmacodynamic (PD) properties. Select a body mass index of 20 kg / m based on history, physical examination, ECG, and routine laboratory tests (eg, blood, chemistry, blood count, urinalysis, and drug screen) 2 with 30kg / m 2 Healthy adult male subjects (approximately 18 to 55 years of age) were used in the study. The half-life extended GLP-1 / GIP co-agonist peptide tested in all examples was SEQ ID NO:153.
[0176] The subjects were randomly divided into several teams. Cohorts 1, 2, 3, 4, 5, and 6 each consisted of 6 patients receiving 0.1 mg, 0.3 mg, 1 mg, 2 mg, 4 mg, and 8 mg of the half-life-extended GLP-1...
Embodiment 2
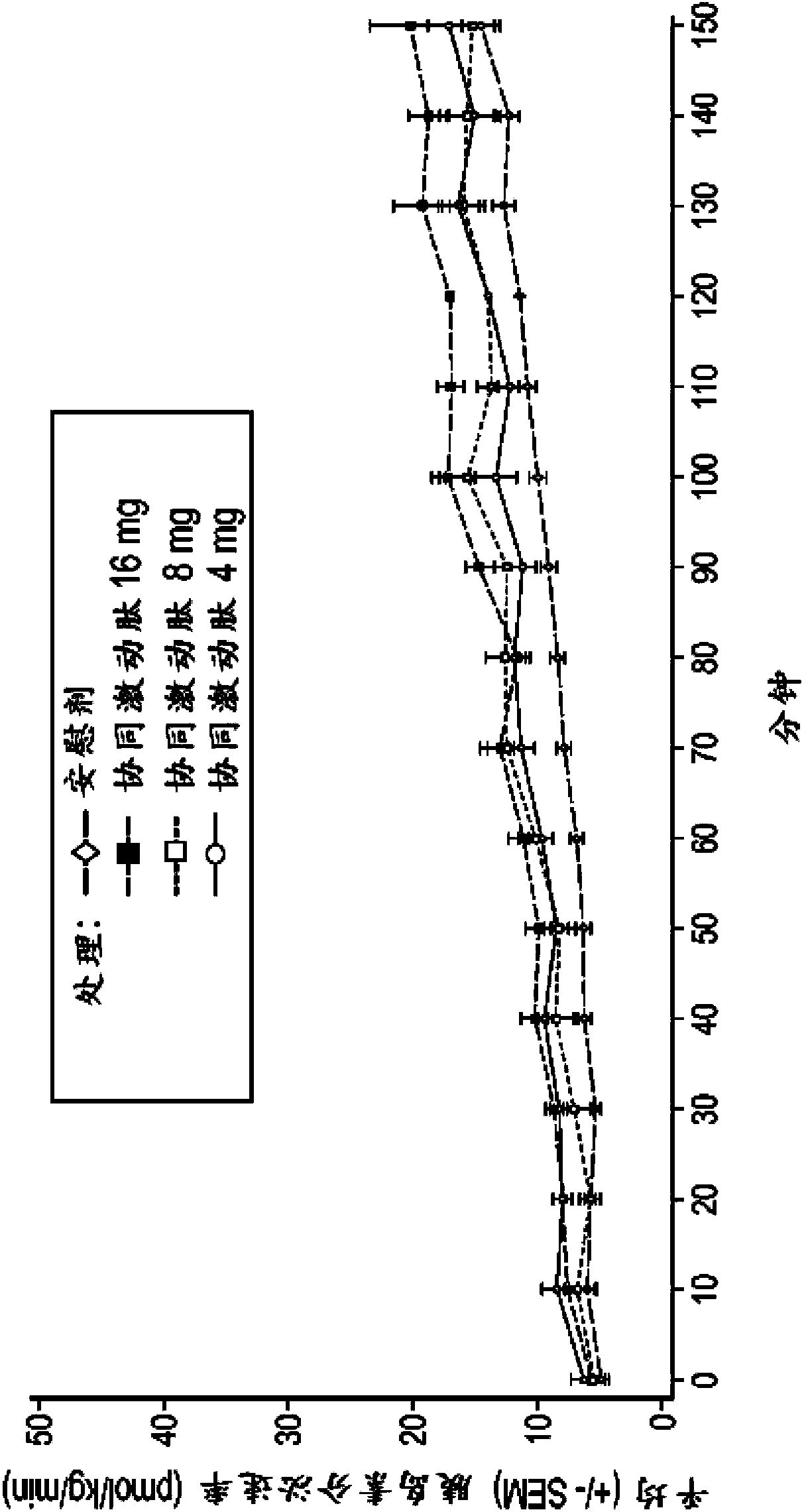
[0182] Example 2: GLP-1 / GIP co-agonist peptides with extended half-life increase insulin secretion in a dose-dependent manner
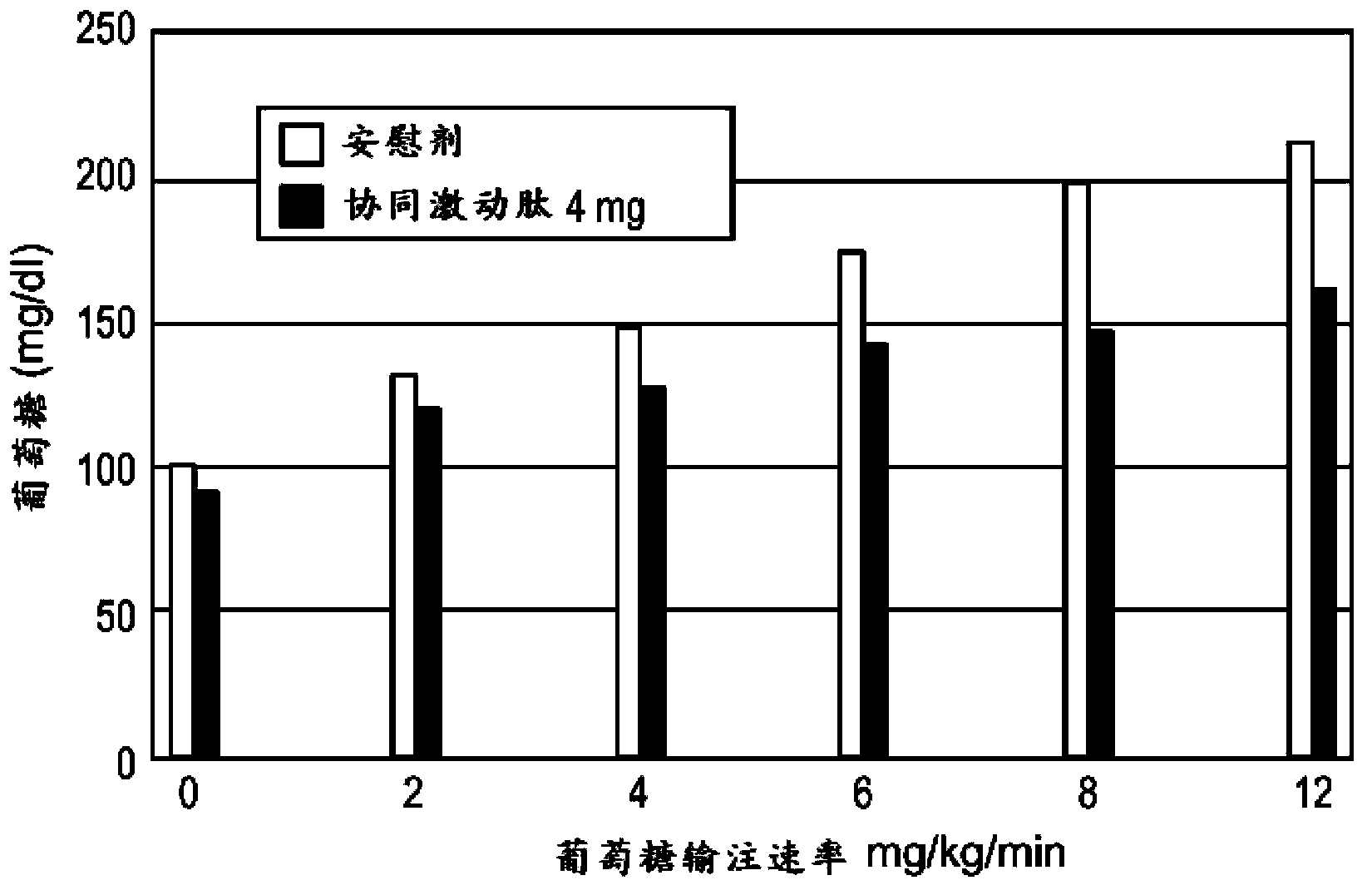
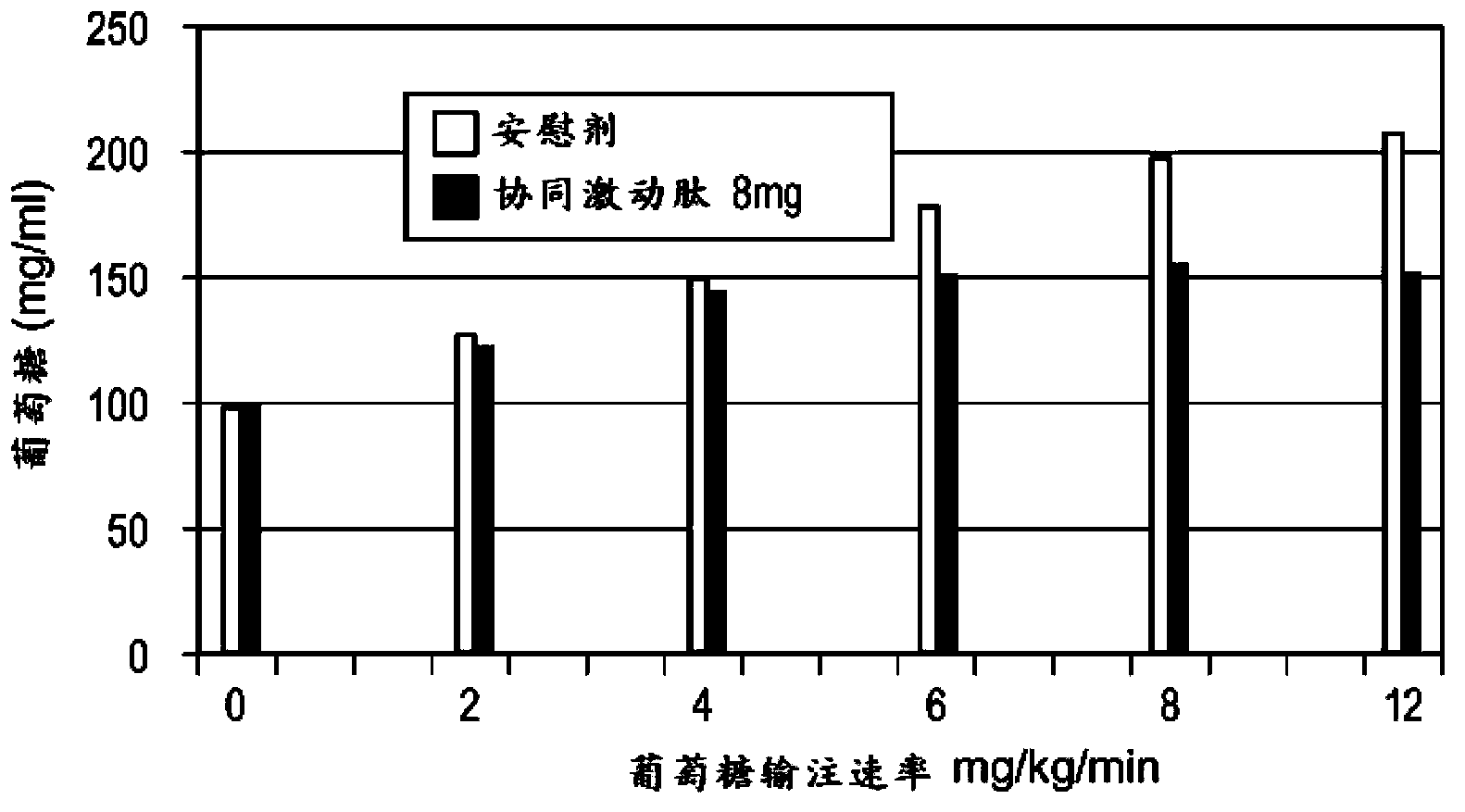
[0183] Phase I, randomized, placebo-controlled, active-controlled two-arm study in healthy male and female subjects to assess the effect of a range of doses on the β-cell response to a glucose load and to assess the effect of these doses on gastric emptying . β-cell function was assessed by calculating prehepatic insulin secretion in response to gradient glucose infusion. Gastric emptying was assessed by measuring the plasma profile of absorbed acetaminophen.
[0184] Subjects in Group 1 (Cohort 1) received two abdominal subcutaneous (SC) injections of placebo 2 hours apart on Day 1, followed by BYETTA (Amylin Pharmaceuticals) two 5 μg abdominal subcutaneous injections (SC). In Arm 2, subjects in Cohort 2 received placebo on day 1, followed by 8 mg of a half-life-extended GLP-1 / GIP co-agonist peptide on day 2. Subjects in Cohort 3 received placebo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com