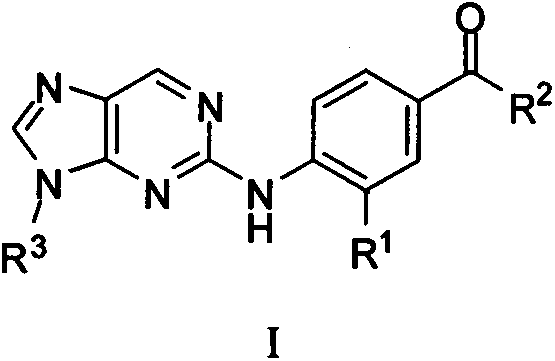
2-phenylaminopurine plk1 (polo-like kinase 1) inhibitors and applications thereof
An amino and purine technology, applied in the field of Polo-like kinase 1 inhibitor, 2-phenylaminopurine derivatives, can solve the problems of tumor cell bipolar spindle formation obstruction, apoptosis, growth inhibition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
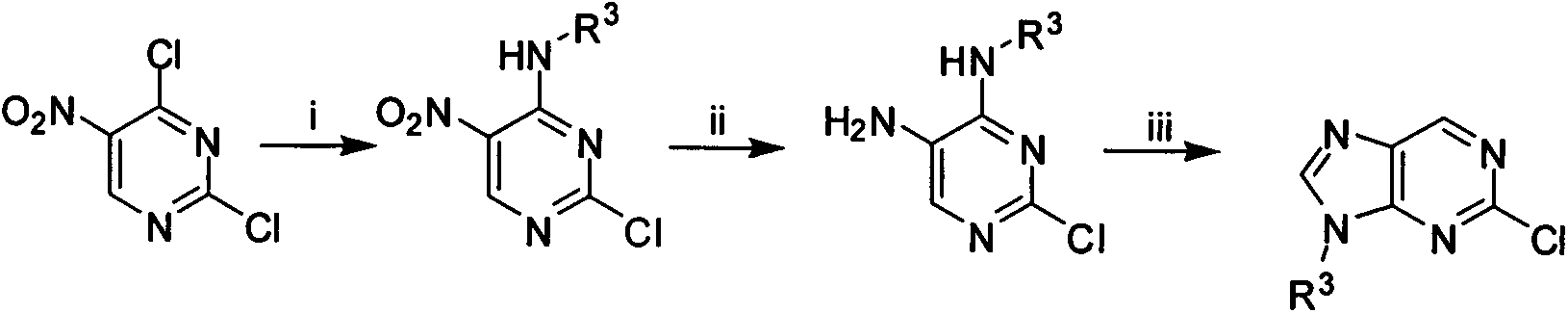
[0089] 2-Chloro-9-cyclopentyl-9H-purine (M1)
[0090] Add 5g (25.8mmol) of 2,4-dichloro-5-nitropyrimidine and 2ml THF into a 50ml reaction bottle, stir evenly and cool to -40°C, slowly drop in 2.2g cyclopentylamine, react for 4h, and complete; Remove the solvent by steaming under pressure, add water, extract (50ml×3), column chromatography (DCM:MeOH=100:1), and obtain 4.26g of 2-chloro-4-(N-cyclopentyl)amino 5-nitropyrimidine, Yield 67.9%, MS [M+H] + 243.1.
[0091] Add 2-chloro-4-(N-cyclopentyl)amino 5-nitropyrimidine 9.1g (36.9mmol) and NH 4 Cl 10g (0.18mol), 70% ethanol aqueous solution 100ml, mechanically stirred, slowly added reduced iron powder 10.5g (0.19mol), reflux reaction for 5h. Suction filtration while hot, evaporate the solvent under reduced pressure, extract (50ml×3), and column chromatography (EA:PE=100:1) to obtain 2-chloro-4-(N-cyclopentyl)aminopyrimidine-5- Amine 7.6g, yield 95%, MS [M+H] + 213.1.
[0092] Add 1.04g (4.9mmol) of the compound 2-chloro-4...
Embodiment 2
[0094] 2-Chloro-9-isopropyl-9H-purine (M2)
[0095] The preparation method was similar to M1, and 0.80 g of the sample was obtained with a yield of 67.5%. MS[M+H] + 197.1, MS[M-H] - 195.1.
Embodiment 3
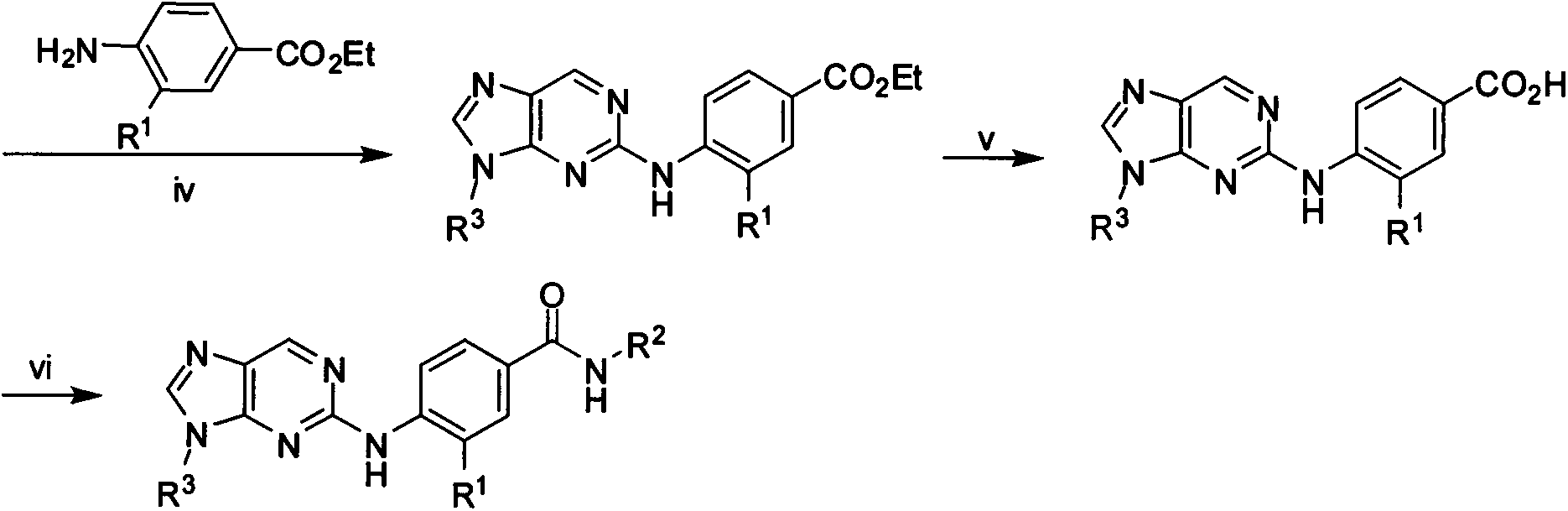
[0097] 4-(9-Cyclopentyl-9H-purin-2-ylamino)benzoic acid (M3)
[0098] Add 10.1g (0.45mmol) of M10.1g (0.45mmol) and 0.07g (0.42mmol) of ethyl p-aminobenzoate into a 25ml eggplant-shaped bottle, stir well, add an appropriate amount of ethylene glycol, and reflux for 24h. After cooling, the reaction solution was poured into a large amount of water, and left to stand, a white milky solid precipitated, which was filtered by suction and dried to obtain 34 mg of a white solid, with a yield of 21.3%.
[0099] Add 0.25g (0.71mmol) of the product from the previous step, 0.08g (2mmol) of KOH, and 10ml of MeOH into a 25ml eggplant-shaped flask, and reflux for 12h. Add 10ml H 2 O, the pH was adjusted to be acidic, and a solid was precipitated. After drying, 0.23 g of a brown solid was obtained, and the yield was 98.33%. MS[M+H] + 324.2, MS[M-H] - 322.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com