New conjugated molecules comprising peptide derived from CD4 receptor coupled to polyanionic polypeptide for treatment of AIDS
A technology of anionic polypeptides and conjugated molecules, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, receptors/cell surface antigens/cell surface determinants, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Embodiment 1: Synthesis of conjugated molecules of the present invention
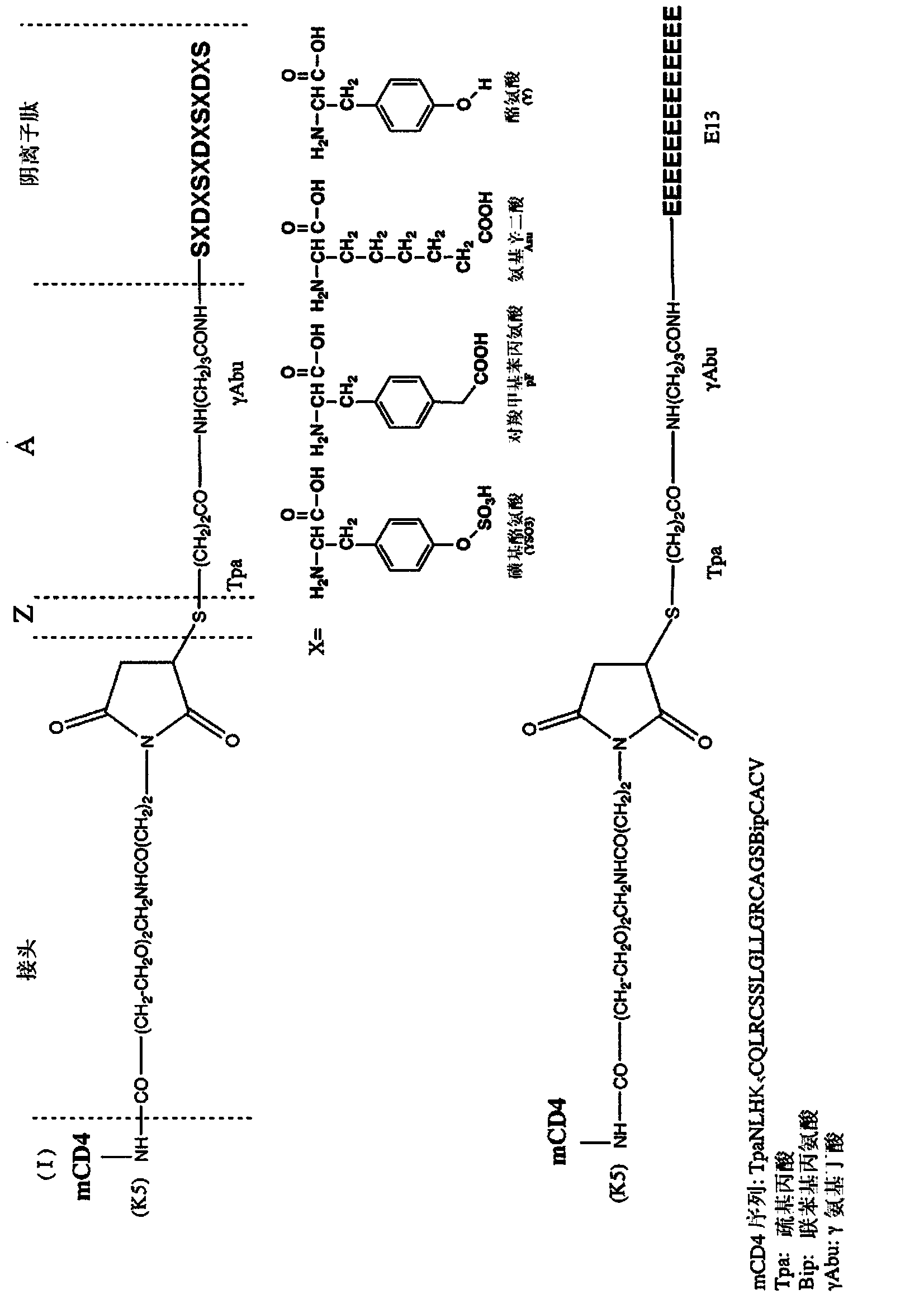
[0166] 1.1 Synthesis of peptides of formula H-γ-Abu-SXDXSXDXSXDXS-OH, wherein X=YSO3 (P3YSO3), Y(P3Y), Asu(P3Asu), or pF(P3pF); H-γ-Abu-SY SO3 Dy SO3 Sy SO3 DYSYDYS-OH (Nter3 Sulfate); H-γ-Abu-SYDYSYDY SO3 Sy SO3 Dy SO3 S-OH (Cter3 Sulfate); and H-γ-Abu-(Glu) 13 -OH(E13); where γ-Abu:NH 2 -(CH 2 ) 3 -CO.
[0167] The following examples describe the synthesis of modified peptides of formula H-γ-Abu-SXDXSXDXSXDXS-OH, wherein X=YSO3(P3YSO3), Y(P3Y), Asu(P3Asu) or pF(P3pF); H-γ-Abu-SY SO3 Dy SO3 Sy SO3 DYSYDYS-OH (Nter3 Sulfate); H-γ-Abu-SYDYSYDY SO3 Sy SO3 Dy SO3 S-OH (Cter3 Sulfate); H-γ-Abu-(Glu) 13 -OH(E13); where γ-Abu:NH 2 -(CH 2 ) 3 -CO. In this formula, the anionic peptides of the present invention (except peptide E13) correspond to SXDXSXDXSXDXS-OH (SEQ ID NO: 19), where X is as above (same or different).
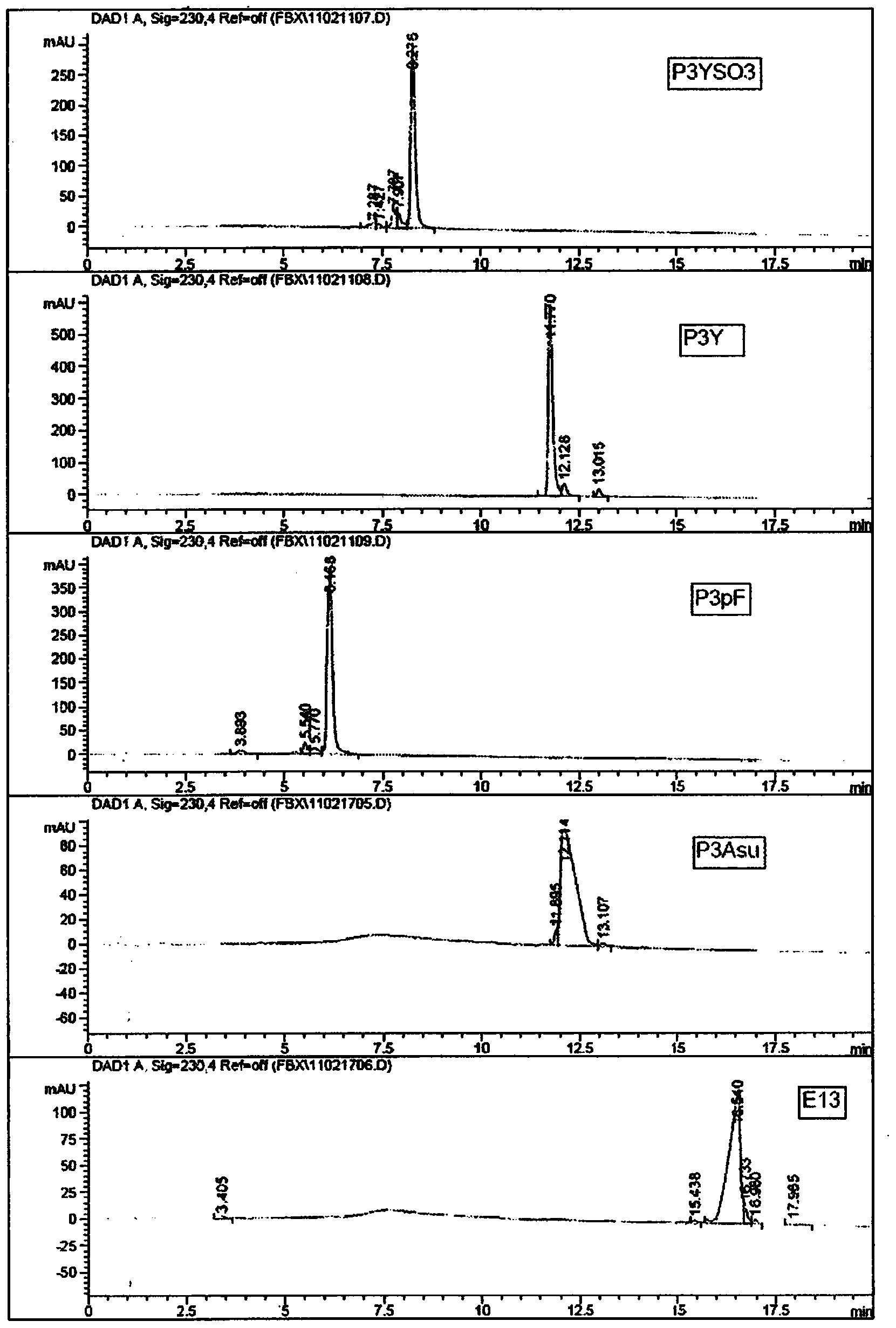
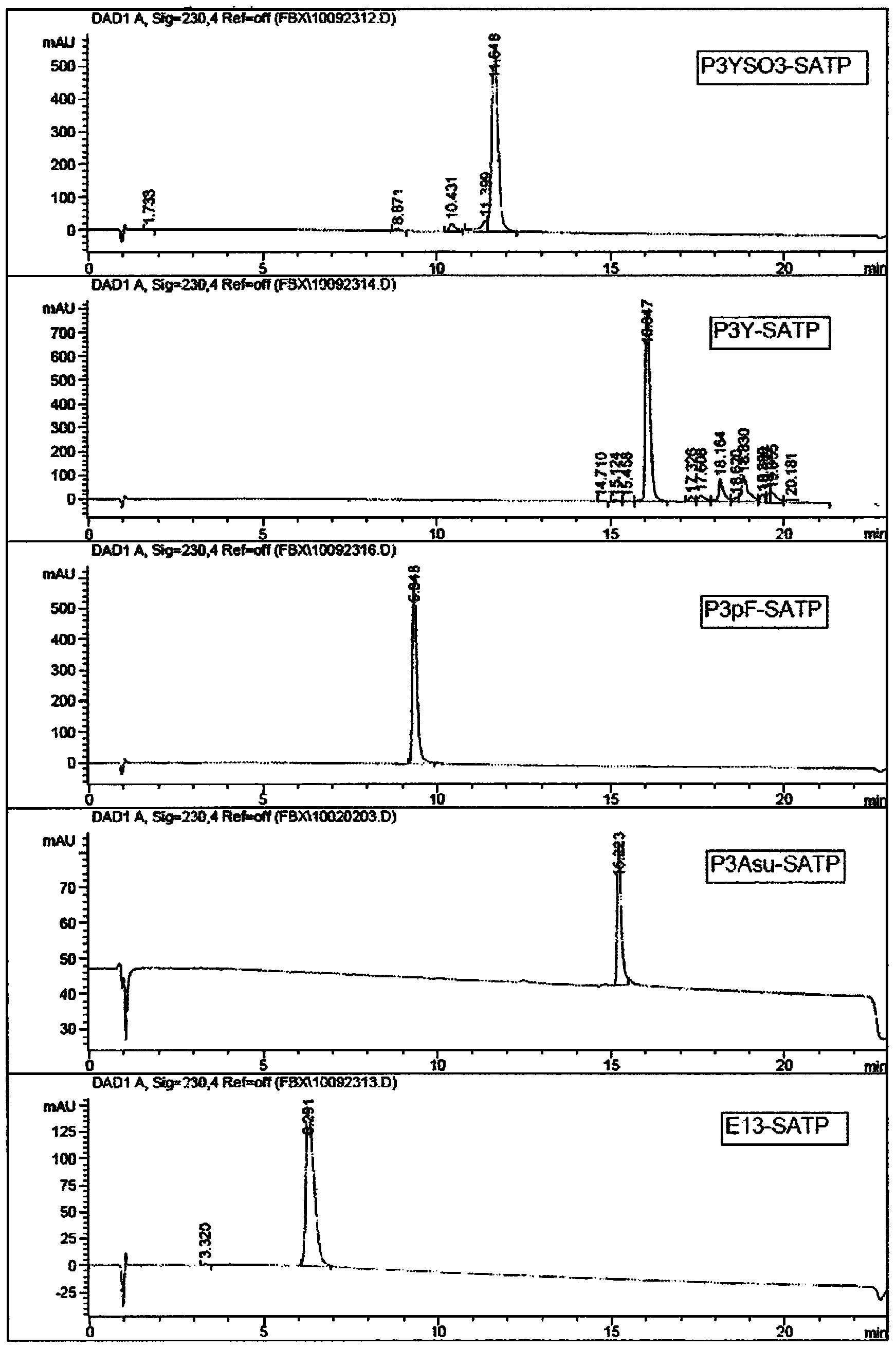
[0168] The resulting modified peptide is:
[0169] P3YSO3 (SEQ I...
Embodiment 2
[0196] Embodiment 2: Biacore evaluation
[0197] 2.1. Operation
[0198] Two different gp120s were employed, derived from an X4-type isolate (gp120MN) and an R5-type isolate (gp120YU2). The ability of different molecules to inhibit the interaction of gp120 with various receptors or co-receptors was measured by surface plasmon resonance (Biacore).
[0199] To this end, proteins (in this case the gp120 envelope protein) can be immobilized on a biosensor (Sensorchip CM4Biacore) according to the procedure described (Vivès et al. J. Biol. Chem. 279, 54327-54333, 2005). surface. When injected onto surfaces coated with the MN(X4) or YU2(R5) gp120 envelope, mCD4 binds and exposes the CD4i epitope on gp120 responsible for the envelope binding of mAb17b ( Figure 5 A) (mAb17b antibody recognizes CD4-induced epitopes and mimics co-receptors CCR5 or CXCR4). The interaction signal between gp120 and mAb17b was measured as a function of time when mCD4 or the compound to be tested was inj...
Embodiment 3
[0211] Embodiment 3: the antiviral activity of peptide of the present invention to X4-tropism HIV-1-LAI and R5-tropism HIV-1 / Ba-L strain
[0212] 3.1. Operation
[0213] Antiviral activity was performed as described in WO / 2009 / 098147 and Nature Chemical Biology, 2009, 5(10), 743-748.
[0214] Briefly, X4-tropic HIV-1-LAI was expanded and titrated in vitro on phytohemagglutinin-P (PHA-P)-activated peripheral blood mononuclear cells (PBMC) (Barre-Sinoussi, Science 220, 868 -71, 1983) or R5-tropic HIV-1 / Ba-L (Gartner et al., Science 233, 215-9, 1986) strain. use The formula for calculating the tissue culture infection dose ( Arch. Exp. Path. Pharmak. 162, 480-483, 1931). For antiviral assays, PHA-P-activated PBMCs were pretreated for 30 min with six concentrations of each drug (diluted 1:5 between 0.5 μM and 160 pM) and treated with X4-tropic LAI or R5-tropic Ba - Infection with one hundred and 50% tissue culture infectious dose (TCID50) of the L strain. Drugs were maintai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com