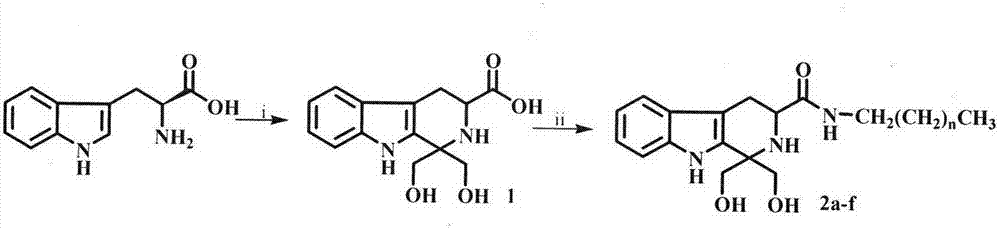
Tetrahydro-beta-carbolinyl-3-formyl aliphatic chain amines, and preparation, nano structure, immunosuppression action and application thereof
An immunosuppressive, fatty amine technology, applied in the field of biomedicine, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
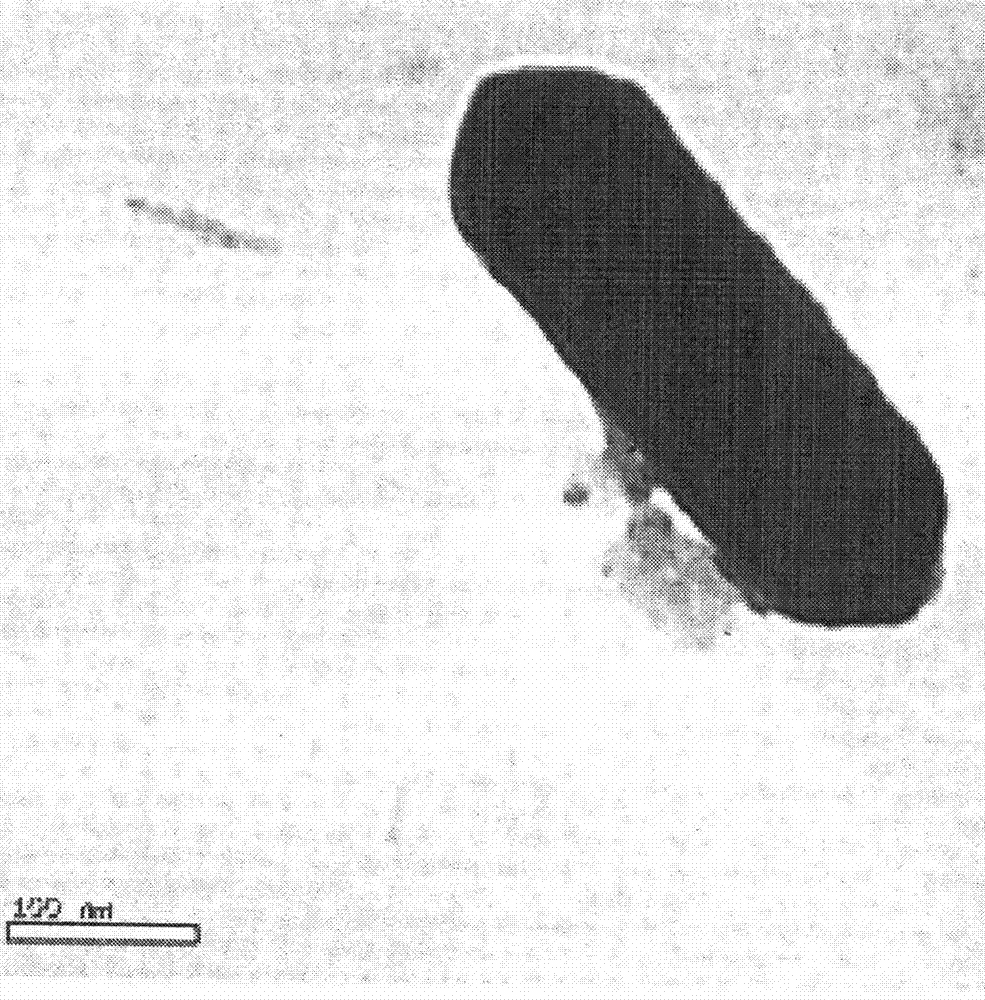
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of 3S-1,1-dimethylol-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid (1)
[0025] Add 6.12 g (30 mmol) of L-tryptophan to 100 mL of water, slowly add concentrated sulfuric acid dropwise in an ice-water bath until the tryptophan is completely dissolved, and then add 3.24 g (36 mmol) of 1,3-dihydroxyacetone. After 24 hours, the reaction solution became turbid, and a solid substance was observed to precipitate out. It was filtered under reduced pressure and washed with distilled water several times to obtain 5.73 g (69%) of the title compound. ESI-MS(m / e): 275[M-H] -
Embodiment 2
[0026] Example 2 Preparation of N-(3S-1,1-dimethylol-2,3,4,9-tetrahydro-β-carboline-3-formyl)-NHC 8 h 17 (2a)
[0027] 1.38g (5.0mmol) 3S-1,1-dimethylol-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid and 1.29g (6.0mmol) CH 3 (CH 2 ) 6 CH 2 NH 2 Dissolve in 20ml of dichloromethane, sonicate. To the resulting solution were added 0.75 g (5.0 mmol) of N-hydroxybenzotriazole (HOBt) and 1.55 g (4.50 mmol) of dicyclohexylcarbodiimide (DCC) under ice cooling. Adjust the pH to 8-9 with N-methylmorpholine (NMM) in an ice bath. Stirred under ice bath for 1 h, then stirred at room temperature for 12 h, TLC (dichloro / methanol=20:1) showed that the product was formed. Dicyclohexylurea (DCU) was filtered off, and 50ml of dichloromethane was added to the filtrate. The resulting solution was sequentially washed with saturated NaHCO 3 Washing with aqueous solution, washing with saturated NaCl aqueous solution, 5% KHSO 4 Washing with aqueous solution and saturated NaCl aqueous solut...
Embodiment 3
[0028] Example 3 Preparation of N-(3S-1,1-dimethylol-2,3,4,9-tetrahydro-β-carboline-3-formyl)-NHC 10 h 21 (2b)
[0029] According to the method of embodiment 2 by 1.38g (5.0mmol) 3S-1,1-dimethylol-2,3,4,9-tetrahydro-beta-carboline-3-carboxylic acid and 1.17g (7.5mmol )CH 3 (CH 2 ) 8 CH 2 NH 2 Obtained 0.32 g (15%) of the title compound as a colorless gum. ESI-MS(m / e): 415[M-H] - .Mp 127-129℃. 1 H-NMR (DMSO-d 6 , 300MHz): δ / ppm=10.58(s, 1H), 8.02(s, 1H), 7.38(d, J=6.0Hz, 1H), 7.32(d, J=3.0Hz, 1H), 7.01(dd, J=6.0Hz, J=3.0Hz, 1H), 6.93(dd, J=3.0Hz, J=6.0Hz, 1H), 4.72-4.62(m, 2H), 3.86-3.83(m, 1H), 3.64- 3.62(m, 1H), 3.58-3.53(m, 3H), 3.14-3.10(m, 2H), 2.88-2.84(m, 1H), 1.52-1.39(m, 2H), 1.38-1.18(m, 14H ), 0.87(t, J=3.0Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com