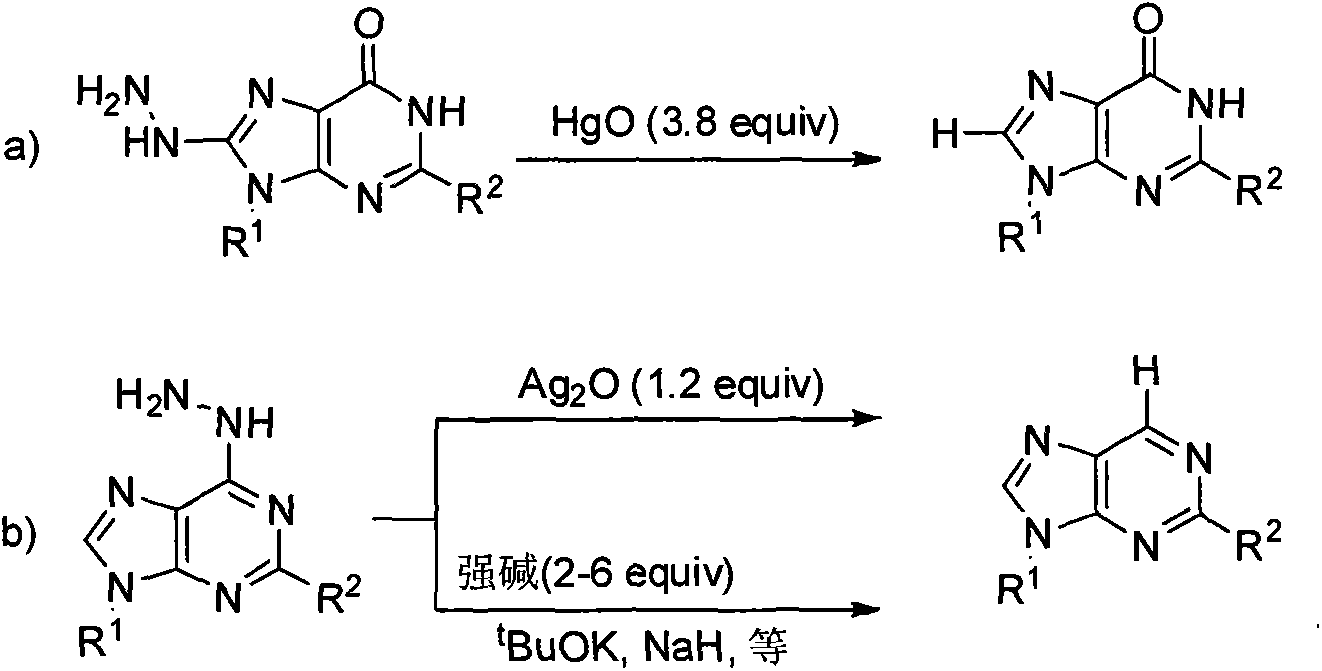
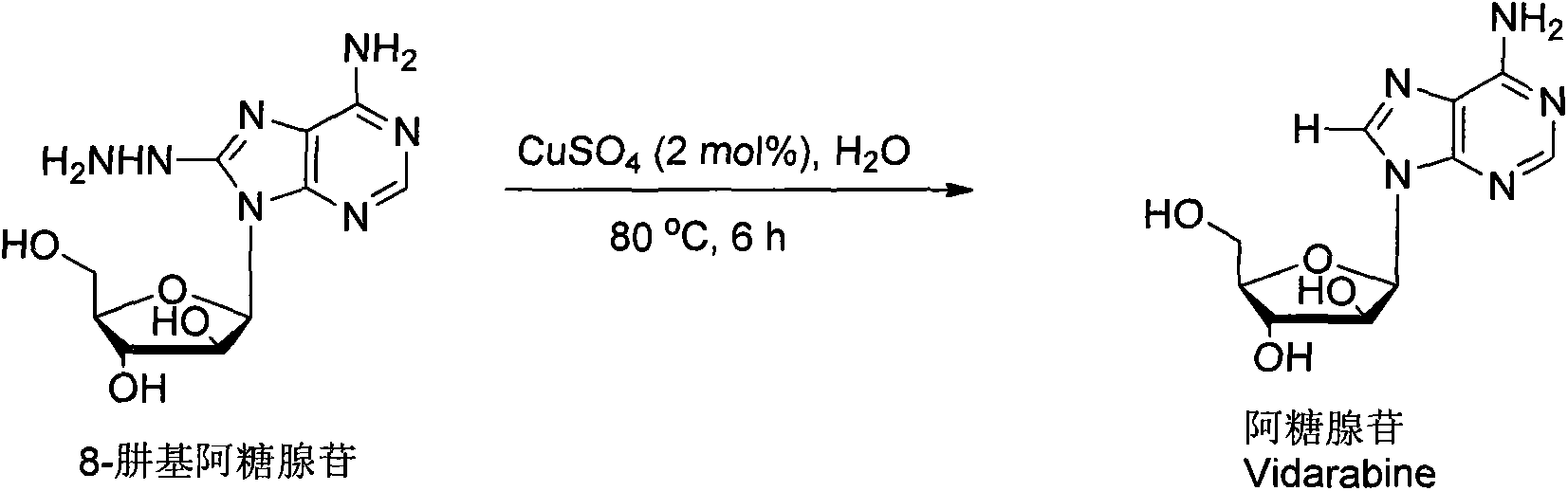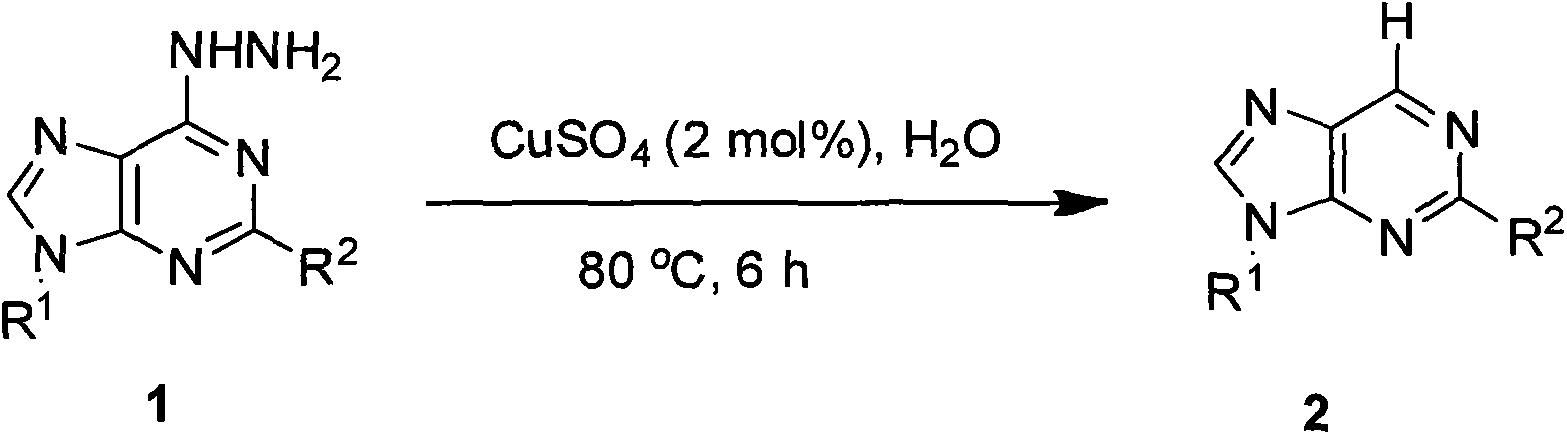Vidarabine and Vidarabine analogues synthesized by air oxidation hydrazine removal method
A compound, hydrazine-based technology, applied in the fields of chemistry and medicine, can solve problems such as high cost, reduced yield, and health hazards of environmental workers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 0.0008g (2mol%) of copper sulfate catalyst, 8-hydrazinoarabinosine (0.075g, 0.25mmol), 3mL of water into a 25mL round-bottom flask, heat to 80℃ in an oil bath, and react for 6 hours, followed by TLC After the reaction is terminated, the solvent is removed in vacuo, and then the target compound arabinosine is obtained by column chromatography with a yield of 87% (yield is based on 8-hydrazinoarab). The product is a white solid.m.p.264-266℃. 1 H NMR(DMSO-d 6 , 400MHz) δ 8.18 (s, 1H), 8.12 (s, 1H), 7.24 (s, 2H), 6.25 (d, J=4.4Hz, 1H), 5.64 (d, J=4.4Hz, 1H), 5.56(t, J=3.6Hz, 1H), 5.13(t, J=4.4Hz, 1H), 4.13(brs, 2H), 3.79-3.76(m, 1H), 3.69-3.61(m, 2H); 13 C NMR(DMSO-d 6 , 100MHz) δ156.3, 152.9, 149.8, 140.8, 118.7, 84.5, 84.0, 76.1, 75.4, 61.3; HRMS calcd for C 10H13 N 5 NaO 4 [M+Na + ]290.0860, found290.0857.
Embodiment 2
[0020] Add 1.1g (2mol%) of copper sulfate catalyst, 8-hydrazinoarabinosine (100g, 0.34mol), 1L of water into a 2L reaction flask, heat the oil bath to 80°C, react for 6 hours, follow the reaction by TLC, After the reaction was terminated, the solvent was removed in vacuo, the activated carbon was decolorized, and then recrystallized with water to obtain the target compound arabinosine with a yield of 77%.
Embodiment 3
[0022] Add 25g (2mol%) of copper sulfate catalyst, 8-hydrazinoarabinosine (1.5Kg, 5mol), 15L of water into a 100L reactor, heat to 80℃, react for 6 hours, follow the reaction with TLC, and terminate the reaction Then, the solvent was removed in vacuo, the activated carbon was decolorized, and then recrystallized with water to obtain the target compound arabinosine with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com