DC (Dendritic Cell) vaccine as well as preparation method and application thereof
A vaccine and a series of technologies are applied in the fields of pharmaceutical formula, particle and sedimentation analysis, blood/immune system cells, etc., which can solve problems such as not easy to obtain fragments, limited clinical application, and unsuitable for clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028]In order to make the present invention clearer, the present invention will be further described in conjunction with the accompanying drawings and embodiments. It should be understood that this embodiment is not intended to limit the protection scope of the present invention.
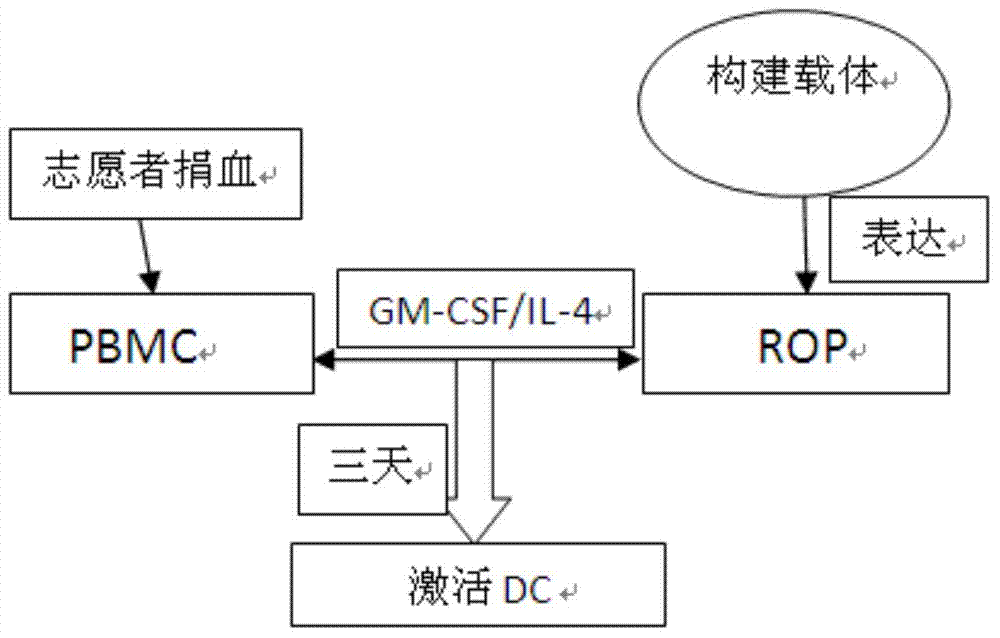
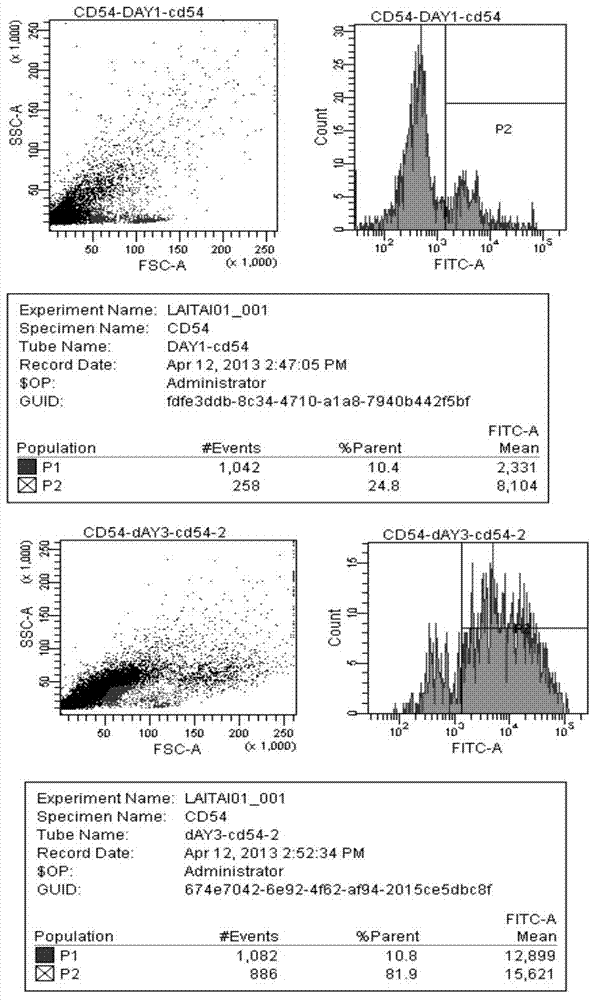
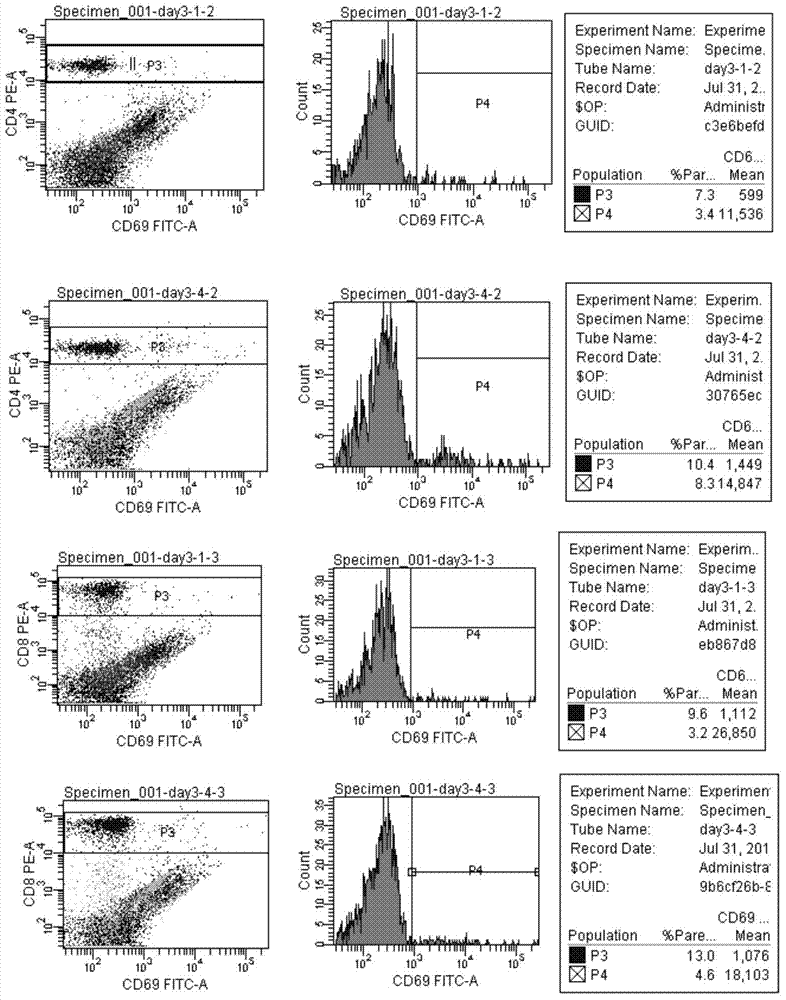
[0029] A new DC vaccine strategy was adopted to construct a recombinant overlapping peptide (ROP) fusion protein according to the amino acid sequence of the tumor antigen. The ROP fusion protein is composed of a series of 15 amino acid polypeptides. The 11 amino acids of the adjacent two polypeptides are completely overlapping and identical. The overlapping peptides are connected by the same restriction site li-key (LRMK). The constructed code TEV gene was subcloned into pET28 vector through NdeI and BamH restriction sites. The overlapping peptide fusion protein was isolated and purified after expression in Escherichia coli. PBMCs isolated from human blood were co-cultured with GM-CSF and ROP fusi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com