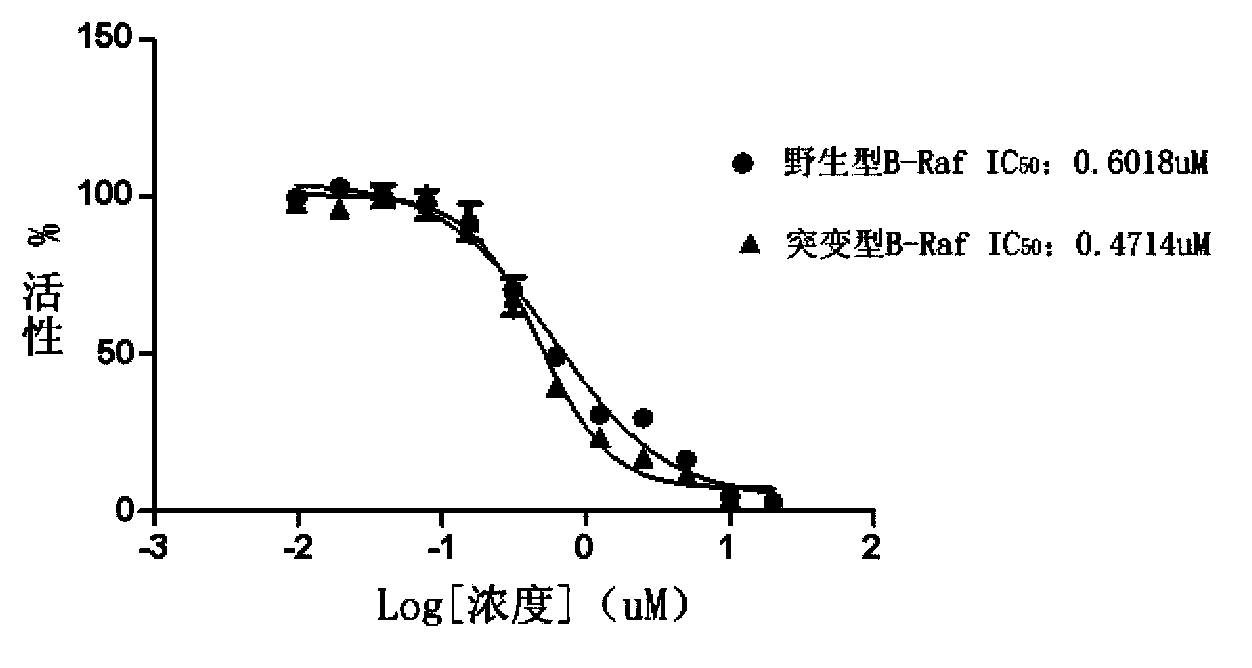
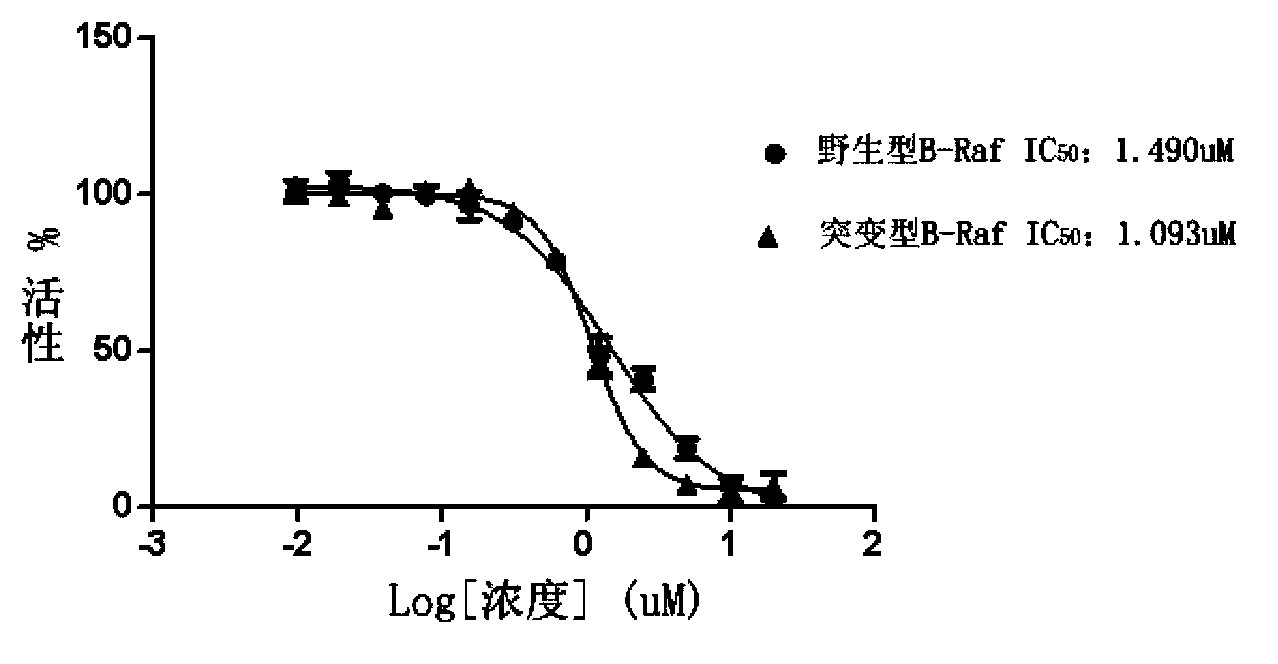
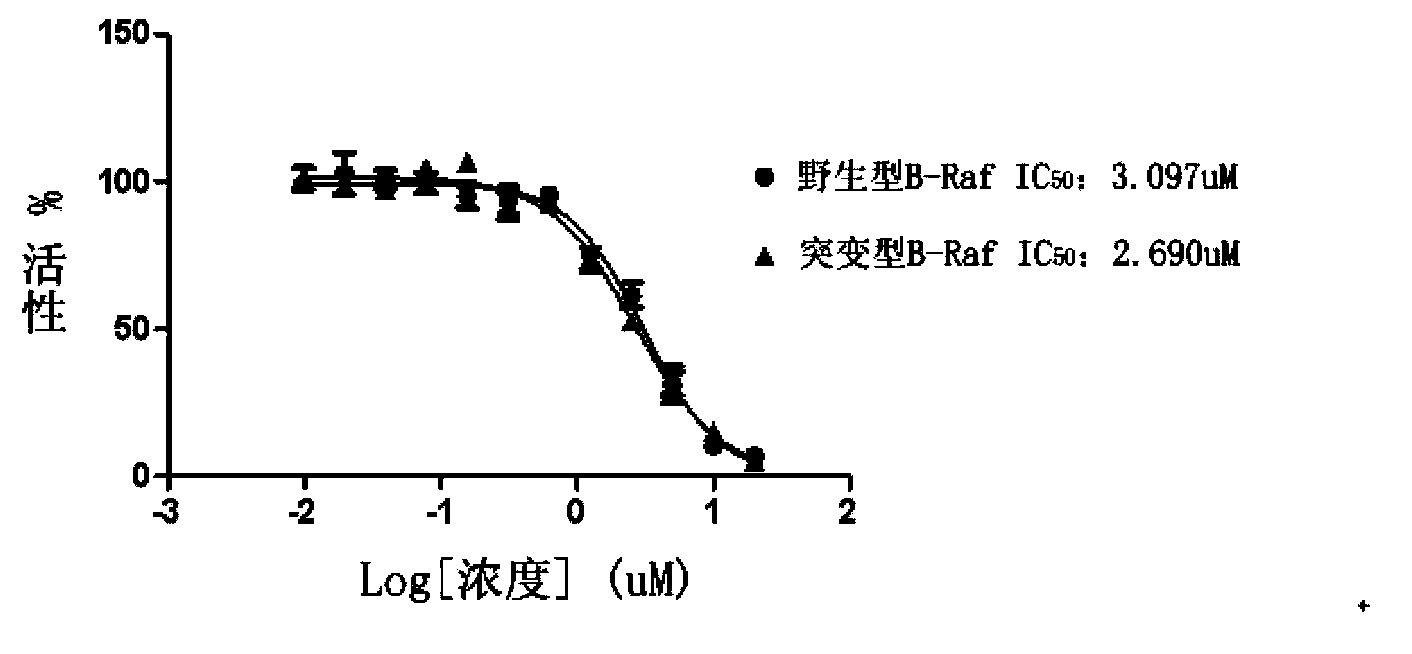
2-substituted-5-phenyl furan compound, and preparation method, pharmaceutical composition and application thereof
A technology of phenylfuran and compounds, applied in the field of preparation of B-Raf kinase inhibitors, can solve problems such as inability to achieve effective concentration, poor bioavailability, and no effect on melanoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Example 1: 5-(5-(4-nitrophenyl)-furan-2-enyl)-2-thiobarbituric acid (BBT01)
[0145] Add 100mg of 5-(4-nitrophenyl)-furan-2-carbaldehyde and 1 equivalent of thiobarbituric acid to 2mL of glacial acetic acid, heat up to 90°C and react for 1-2 hours, there are a lot of orange solids Precipitate. The purer crude product can be obtained by suction filtration, and the crude product is fully washed with methanol to obtain the pure compound 5-(5-(4-nitrophenyl)-furan-2-enyl)-2-thiobarbituric acid 136mg, yield 86%.
[0146] 1 H NMR(400MHz,DMSO)δ12.50(s,1H),12.44(s,1H),8.60-8.58(d,J=4.0Hz,1H),8.48-8.35(d,J=8.5Hz,2H) ,8.24-8.21(d,J=8.6Hz,2H),8.12(s,1H),7.74-7.72(d,J=4.0Hz,1H).ESI-MS m / z:344.0[M+H] + .
Embodiment 2
[0147] Example 2: 5-(5-(4-chlorophenyl)-furan-2-enyl)-2-thiobarbituric acid (BBT02)
[0148] Using 5-(4-chlorophenyl)-furan-2-carbaldehyde instead of 5-(4-nitrophenyl)-furan-2-carbaldehyde as raw material, it was prepared according to the method in Example 1.
[0149] 1 H NMR(400MHz,DMSO)δ12.44(s,1H),12.38(s,1H),8.61-8.60(d,J=4.0Hz,1H),8.11(s,1H),8.03-7.99(d, J=8.6Hz,2H),7.63-7.60(d,J=8.6Hz,2H),7.55-7.53(d,J=4.0Hz,1H).ESI-MS m / z:333.0[M+H] + .
Embodiment 3
[0150] Example 3: 5-(5-(2-nitrophenyl)-furan-2-enyl)-2-thiobarbituric acid (BBT03)
[0151] Use 5-(2-nitrophenyl)-furan-2-carbaldehyde instead of 5-(4-nitrophenyl)-furan-2-carbaldehyde as raw material, and prepare according to the method in Example 1.
[0152] 1 H NMR(400MHz,DMSO)δ12.51(s,1H),12.45(s,1H),8.61-8.60(d,J=4.0Hz,1H),8.09-8.03(t,J=8.3Hz,2H) ,7.89(s,1H),7.88-7.83(t,J=8.3Hz,1H),7.78-7.74(t,J=8.3Hz,1H),7.42-7.40(d,J=4.0Hz,1H). ESI-MS m / z:344.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com