Content and impurity measuring method for tamibarotene and preparation thereof
A technology for the determination of tamibarotene and impurities, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems of not being able to control the quality of tamibarotene and its preparations, and affecting the production and application of tamibarotene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
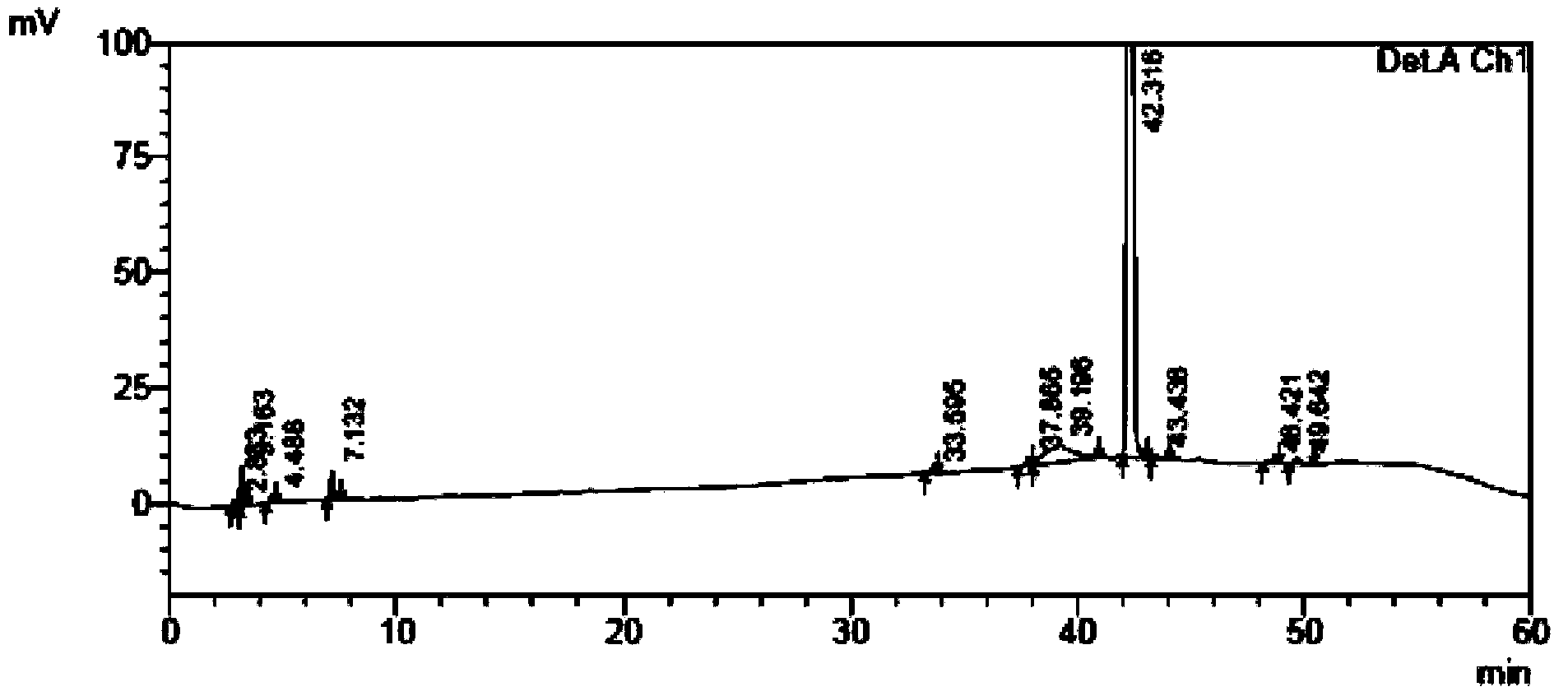
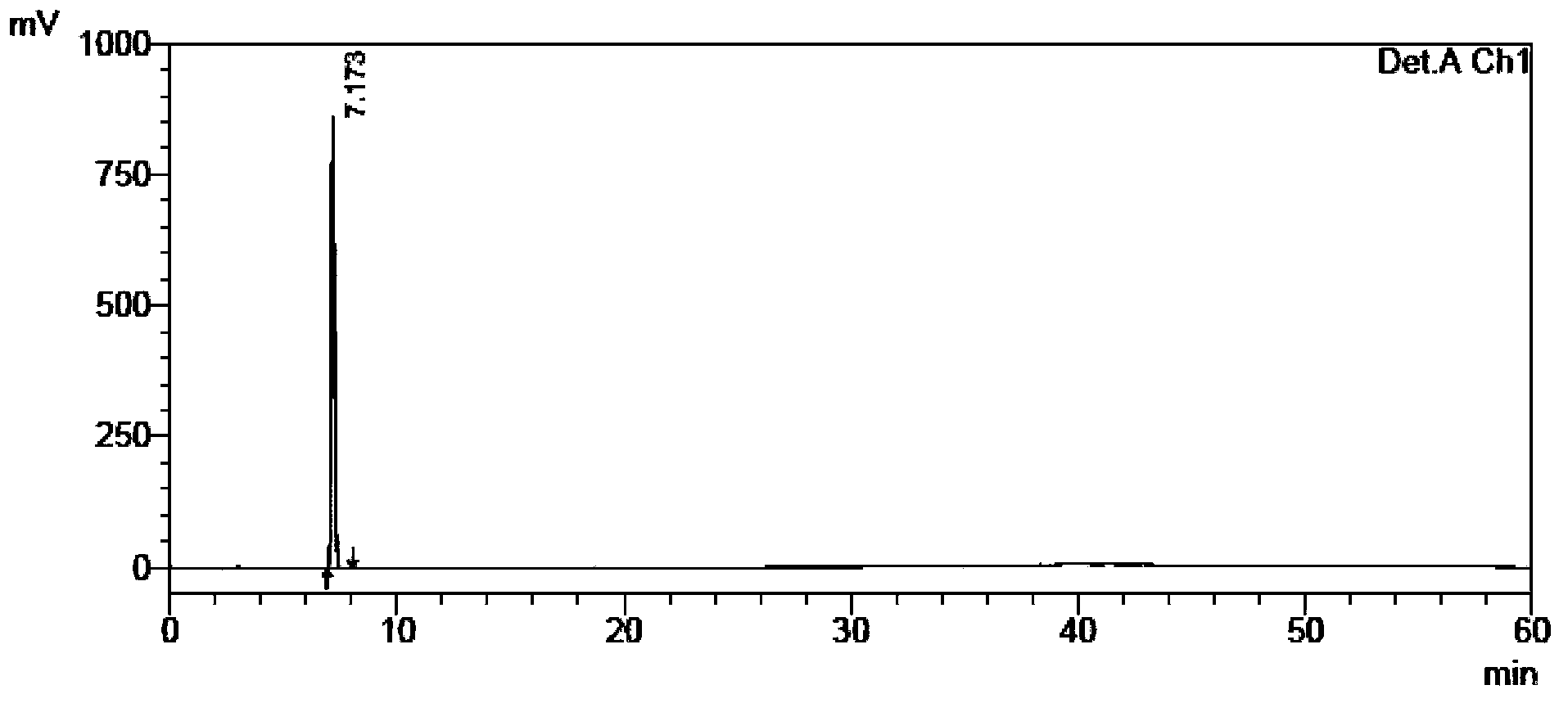
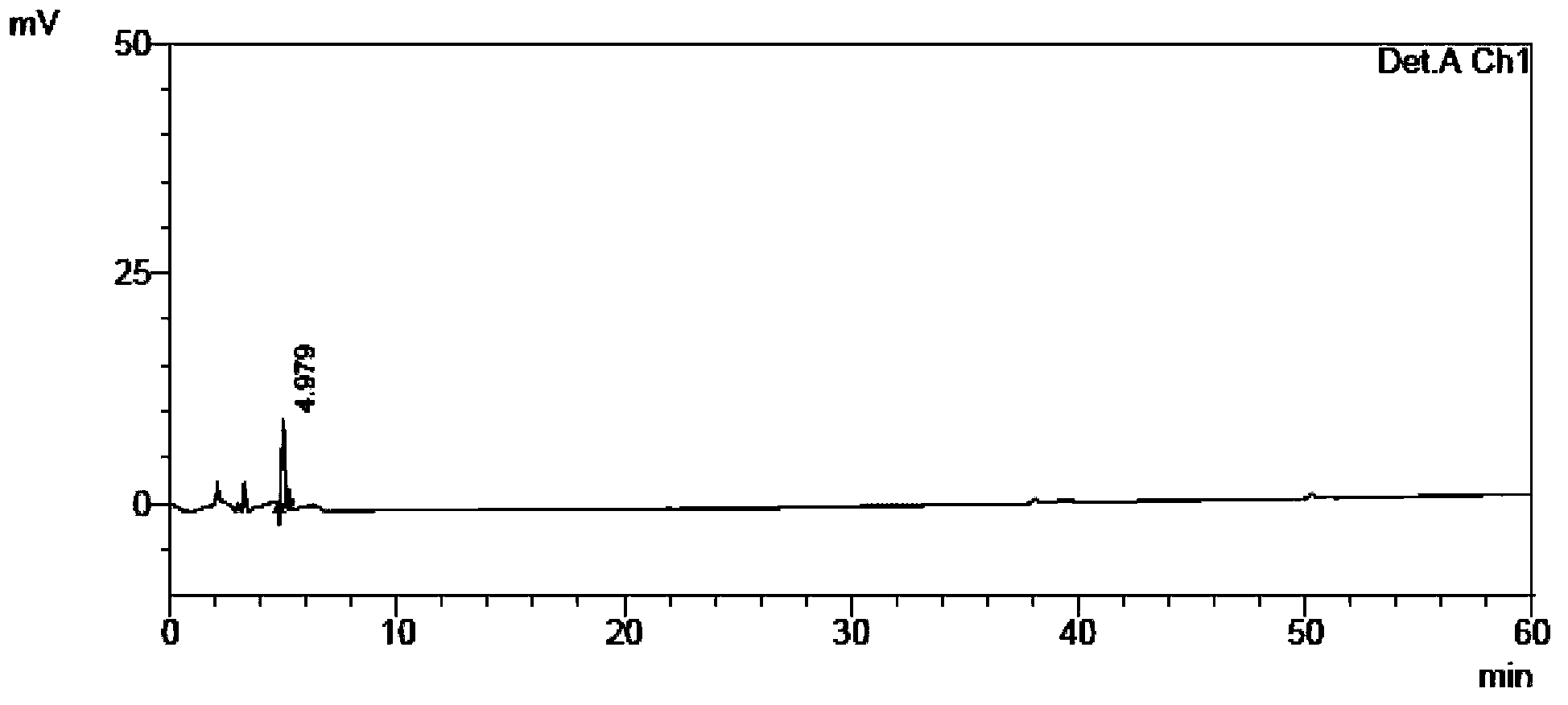
[0115] The self-made crude tamibarotene and impurity 1, impurity 2, and impurity 3 were used as samples to be tested.
[0116] Chromatographic conditions: chromatographic column with octadecylsilane bonded silica gel as filler; detection wavelength 240nm; column temperature 30°C; phosphate buffer solution with a volume ratio of water to phosphoric acid of 2000:1 as mobile phase A, and acetonitrile as mobile phase B Phase, where the gradient elution program of mobile phase A and mobile phase B is:
[0117] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
70
30
20
55
45
40
30
70
60
30
70
[0118] Sample solution configuration: Take an appropriate amount of crude tamibarotene in a volumetric flask, add acetonitrile-water and phosphoric acid with a volume ratio of 2000:1 phosphate buffer solution (60:40) to dissolve, add mobile phase to dilute to the mark, shake uniform...
Embodiment 2
[0122] The self-made crude tamibarotene and impurity 1, impurity 2, and impurity 3 were used as samples to be tested.
[0123] Chromatographic conditions: chromatographic column with octadecylsilane bonded silica gel as filler; detection wavelength 240nm; column temperature 30°C; phosphate buffer solution with a volume ratio of water to phosphoric acid of 2000:5 as mobile phase A, and acetonitrile as mobile phase B Phase, where the gradient elution program of mobile phase A and mobile phase B is:
[0124] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
70
30
20
55
45
40
30
70
60
30
70
[0125] Sample solution configuration: Take an appropriate amount of crude tamibarotene in a volumetric flask, add acetonitrile-water to phosphoric acid with a volume ratio of 2000:5 phosphate buffer solution (60:40) to dissolve, add mobile phase to dilute to the mark, shake uniform,...
Embodiment 3
[0129] The self-made crude tamibarotene and impurity 1, impurity 2, and impurity 3 were used as samples to be tested.
[0130] Chromatographic conditions: chromatographic column with octadecylsilane bonded silica gel as filler; detection wavelength 240nm; column temperature 30°C; phosphate buffer solution with a volume ratio of water to phosphoric acid of 2000:3 as mobile phase A, and acetonitrile as mobile phase B Phase, where the gradient elution program of mobile phase A and mobile phase B is:
[0131] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
70
30
20
55
45
40
30
70
60
30
70
[0132] Sample solution configuration: Take an appropriate amount of crude tamibarotene in a volumetric flask, add acetonitrile-water and phosphoric acid with a volume ratio of 2000:3 phosphate buffer solution (60:40) to dissolve, add mobile phase to dilute to the mark, shake Evenly,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com