Method of preparing lithium borate ores from mixed brine by utilizing natural energy
A technology of mixing brine and natural energy, applied in the direction of lithium sulfate/sulfite, boron oxide, etc., can solve the problems of difficult processing, harsh environment, loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
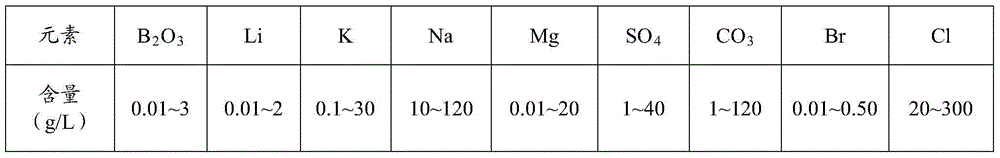
[0062] Example 1: The brine J composition after the above-mentioned evaporation of lithium is: Na + 0.88%, K + 0.38%, Mg 2+ 6.65%, Li + 0.42%, Cl - 20.91%, SO 4 2- 2.08%, B 2 o 3 2.14%, take the above-mentioned brine J and mix it with fresh water or sulphate-type original brine according to the volume ratio of 50%, and carry out natural evaporation under the temperature environment in spring and summer on the plateau to precipitate boron ore. When the boron in the solution is B 2 o 3 The concentration of the meter drops to below 0.5% for separation, and the solution at this time consists of: Na + 6.63%, K + 0.04%, Mg 2+ 2.34%, Li + 0.23%, Cl - 15.61%, SO 42- 3.42%, B 2 o 3 0.47%, the solid phase composition is K + 0.10%, Mg 2+ 6.91%, Li + 0.025%, Cl - 1.83%, SO 4 2- 0.13%, B 2 o 3 30.09%, through physical phase identification and chemical analysis, it is proved that the solid is mainly boronite, and the purity of boron ore reaches more than 90%.
example 2
[0063] Example 2: The composition of brine J after natural evaporation of lithium is: Na + 2.05%, K + 0.43%, Mg 2+ 5.63%, Li + 0.39%, Cl - 19.04%, SO 4 2- 2.67%, B 2 o 3 2.36%, the brine J is mixed with fresh water or sulphate-type original brine according to the volume ratio of 500%, and is naturally evaporated under the temperature environment in spring and summer on the plateau to precipitate boron ore. When the boron in the solution is in the form of B 2 o 3 Separation when the concentration of the meter drops below 0.5%, the solution at this time consists of: Na + 6.01%, K + 0.56%, Mg 2+ 2.13%, Li + 0.33%, Cl - 15.40%, SO 4 2- 2.89%, B 2 o 3 0.50%, the solid phase composition is K + 0.11%, Mg 2+ 7.03%, Li + 0.023%, Cl - 1.93%, SO 4 2- 0.13%, B 2 o 3 30.59%, through physical phase identification and chemical analysis, it is proved that the solid is mainly boronite, and the purity of boron ore reaches more than 90%.
[0064] After the above eleven s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com