A method for preparing transgenic animals capable of expressing human antibodies
A human antibody and gene technology, applied in the use of vectors to introduce foreign genetic material, recombinant DNA technology, animal husbandry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
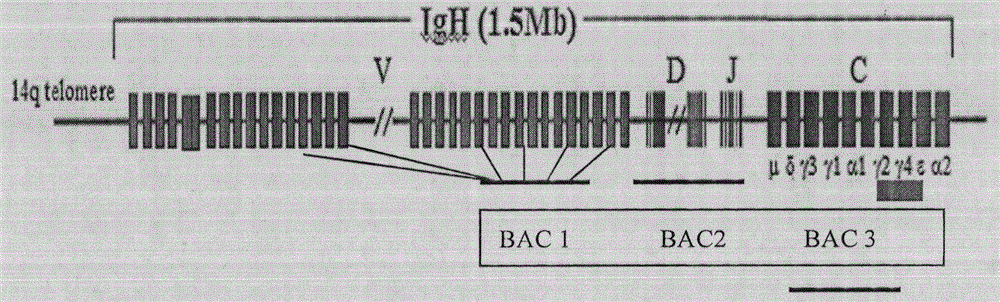
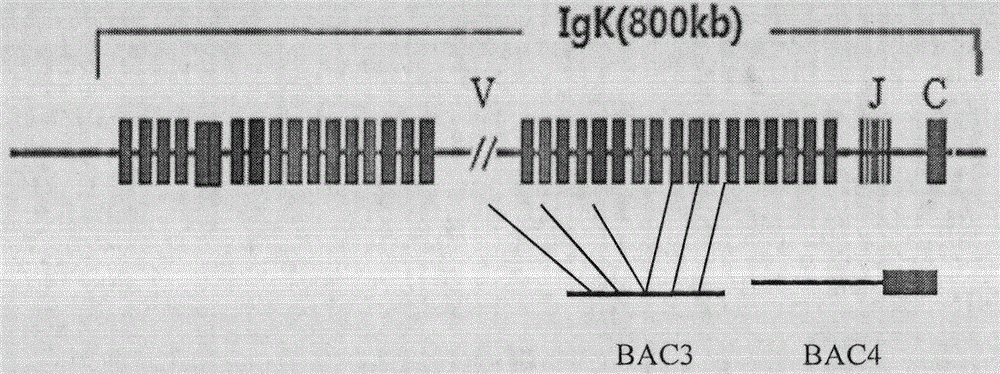
[0053] Purification of BAC DNA Containing Human Antibody Loci
[0054] 1. Small culture of BAC bacteria. Pick a streaked single colony and inoculate it into a 15mL centrifuge tube with a culture volume of 3mL and the cap of the tube is slightly loose. 200rpm, culture at 30°C for 16-20h. The final concentration of chloramphenicol was 12.5ug / mL.
[0055] 2. Large-scale cultivation of BAC strains. Inoculate the small-cultured bacterial solution into a shaker flask at a ratio of 1:500, culture in a volume of 400 mL, 200 rpm, and incubate at 30° C. for 16-20 h. The final concentration of chloramphenicol was 12.5ug / mL.
[0056] 3. Use the MN kit to extract BAC DNA. For related steps, refer to "Endoto-free Plasmid DNA Purification User Manual--Nucleobond Xtra Midi EF". Store BAC DNA at 4°C.
[0057] 4. Use the Nanodrop to measure the concentration of the extracted BAC DNA. PFGE analysis.
[0058] 5. The purified BAC DNA can be used for microinjection directly, or after linea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com