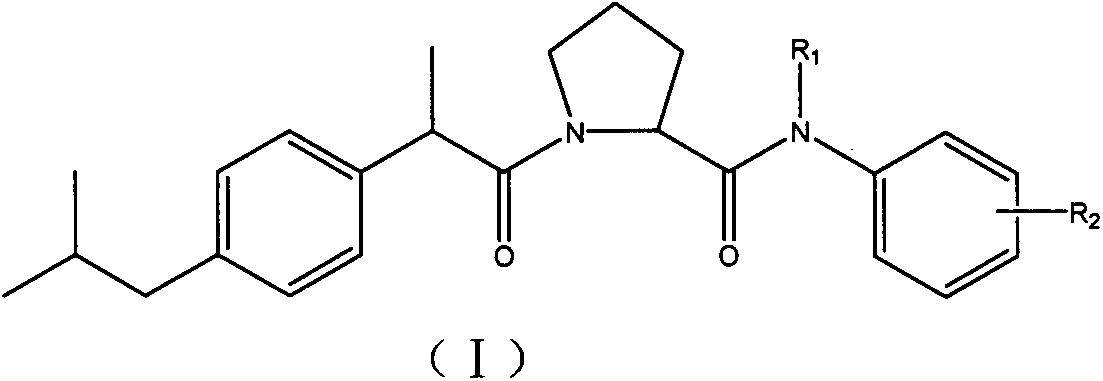
Transient receptor potential vanilloid 1/epoxidase 2(TRPV1/COX-2) dual inhibitor, and preparation method and application thereof in preparation of analgesic medicament
A technology of drugs and medicinal salts, applied in the field of medicinal chemistry, can solve problems such as elevated body temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
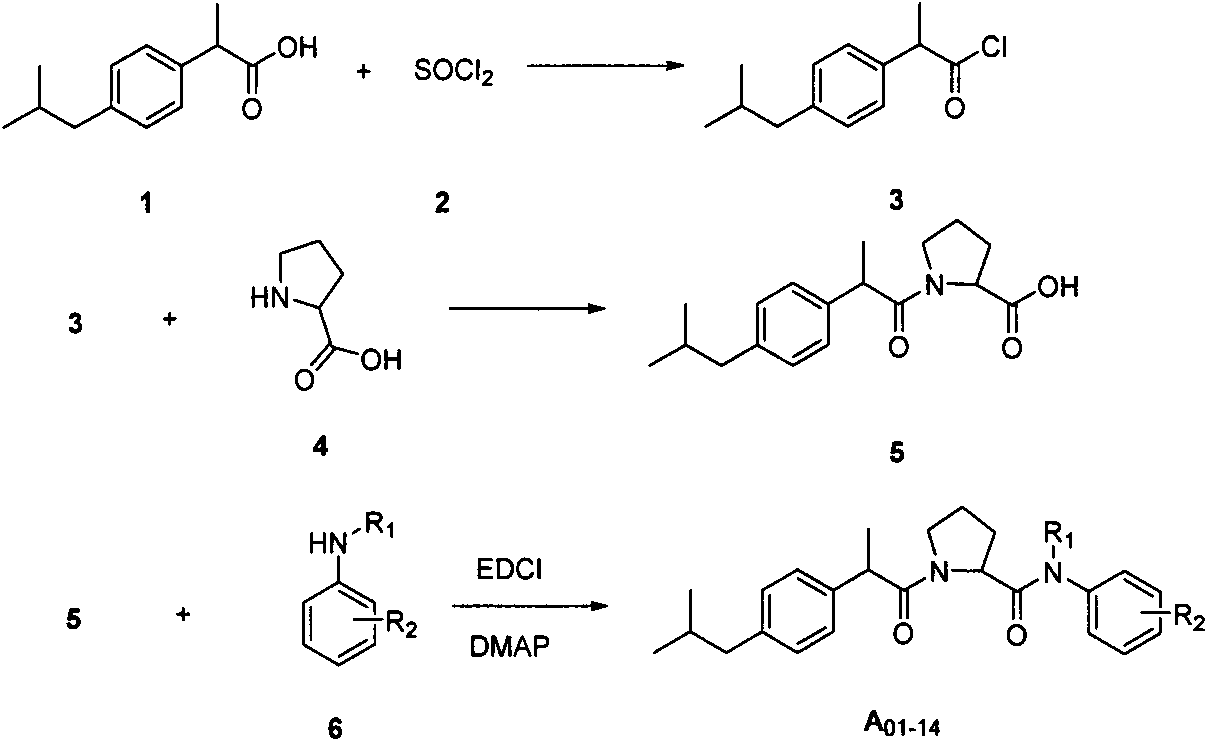
Embodiment 1
[0074] 2-[(4-isobutylphenyl)propionyl]proline (5)
[0075] In a 100ml round bottom flask, 9.62g (46.7mmol) of ibuprofen was dissolved in 20ml of anhydrous dichloromethane, and stirred under ice-water bath conditions. Dissolve 20 g (280.7 mmol) of thionyl chloride in 50 ml of dichloromethane, add 2 drops of DMF, and drop into the reaction flask under ice-salt bath conditions. After dripping and heating to reflux for 3 h, the excess thionyl chloride was evaporated under reduced pressure, and carried twice with toluene to obtain a yellow oil, i.e. acid chloride 3, which was directly used in the next reaction.
[0076] In a 100ml round-bottomed flask, 5.37g (46.7mmol) of proline was dissolved in 26ml of 2N aqueous sodium hydroxide solution, and stirred in an ice bath. Acid chloride 3 and 12ml of 4N aqueous sodium hydroxide solution were added dropwise into the reaction flask alternately. After dripping, the reaction was continued for 1 h under the condition of ice bath, and then...
Embodiment 2
[0078] 1-[(2-(4-isobutylphenyl)propionyl]-2-(N-o-methylphenylformamide)pyrrolidine (A 01 )
[0079] In a 25ml round bottom flask, 51g (3.28mmol) of the compound was dissolved in 5ml of dichloromethane, and stirred in an ice-salt bath. 1.26g (6.56mmol) of EDCI and DMAP (catalytic amount) were dissolved in 10ml of dichloromethane, added dropwise to the reaction solution, and the reaction was continued for 0.5h under ice-bath conditions after dropping. 0.35 g (3.29 mmol) of o-methoxyaniline was dissolved in 10 ml of dichloromethane, and added dropwise to the reaction solution. After the dropwise addition was completed for 0.5 h, the ice bath was removed and the reaction was carried out at room temperature for 12 h. The next day, the TLC reaction was complete, washed with 1N dilute hydrochloric acid (10ml×2), washed with saturated brine (10ml×2), washed with saturated sodium bicarbonate solution (10ml×2), washed with saturated brine (10ml×2), no water and sodium sulfate to dry....
Embodiment 3
[0084] 1-[(2-(4-isobutylphenyl)propionyl]-2-[N-(4-tert-butylphenyl)formamide]pyrrolidine (A 02 )
[0085] Refer to A 01 The preparation of compound 51.00g (3.28mmol), EDCI1.26g (6.56mmol), DMAP (catalytic amount), p-tert-butylaniline 0.60g (3.94mmol), all the other operations are the same as IA 01 Preparation of pure product 0.44g, yield: 29.53%, m.p.136-137°C.
[0086] 1 HNMR (DMSO-d 6 , 300MHz): δppm9.73 (s, 1H, NH), 7.49 (d, J=8.49Hz, 2H, Ar-H,), 7.12 (d, J=8.46Hz, 2H, Ar-H,), 7.21 (d, J=7.95Hz, 2H, Ar-H,), 7.12(d, J=7.47Hz, 2H, Ar-H,), 4.79(d, J=7.32Hz, 1H, C-2”pyrr) , 3.80(m, 1H, CH 3 CHC=O), 3.63(m, 1H, C-5"pyrr), 3.30(m, 1H, C-5"pyrr), 2.47(m, 3H, C-3"pyrr and CH 2 CH(CH 3 ) 2 ,), 2.16(m, 1H, C-3"pyrr), 1.85(m, 2H, C-4''pyrr), 1.72(m, 1H, CH(CH 3 ) 2 ), 1.49 (d, J=6.81Hz, 3H, CH 3 CHC=O), 1.31(s, 9H, (CH 3 ) 3 C), 0.92(d, J=6.60Hz, 6H, CH(CH 3 ) 2 ,);
[0087] IR (KBr, cm -1 ): 3301 (v N-H ), 1902, 1636 (v C=O ), 1596, 1534 (aromatic);
[0088] MS (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com