Diphenylethylene type co-crystallization materials with multi-stimulus fluorescence response property and preparation method thereof
A fluorescent response, stilbene technology, applied in the direction of color-changing fluorescent materials, luminescent materials, halogenated hydrocarbon preparation, etc., can solve the problems of unpredictability of luminescent color, complex spatial structure, etc., and achieve the effect of broadening the application space and the scope of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1) Weigh 0.0332g of (1,4’-bis[2-(o-cyanophenyl)vinyl]benzene) and 0.0544g of 4-bromo-2,3,5,6-tetrachlorobenzoic acid respectively;
[0026] 2) Mix the two evenly, put them into a ball mill (MM200 from Laich Company, Germany), add 30 μL of chloroform, and grind for 30 minutes at 20 r / s;
[0027] 3) Dissolve the ground powder in 20mL of chloroform, place it at room temperature, and wait for the solvent to evaporate to obtain a single crystal product.
[0028] 4) Characterize the product:
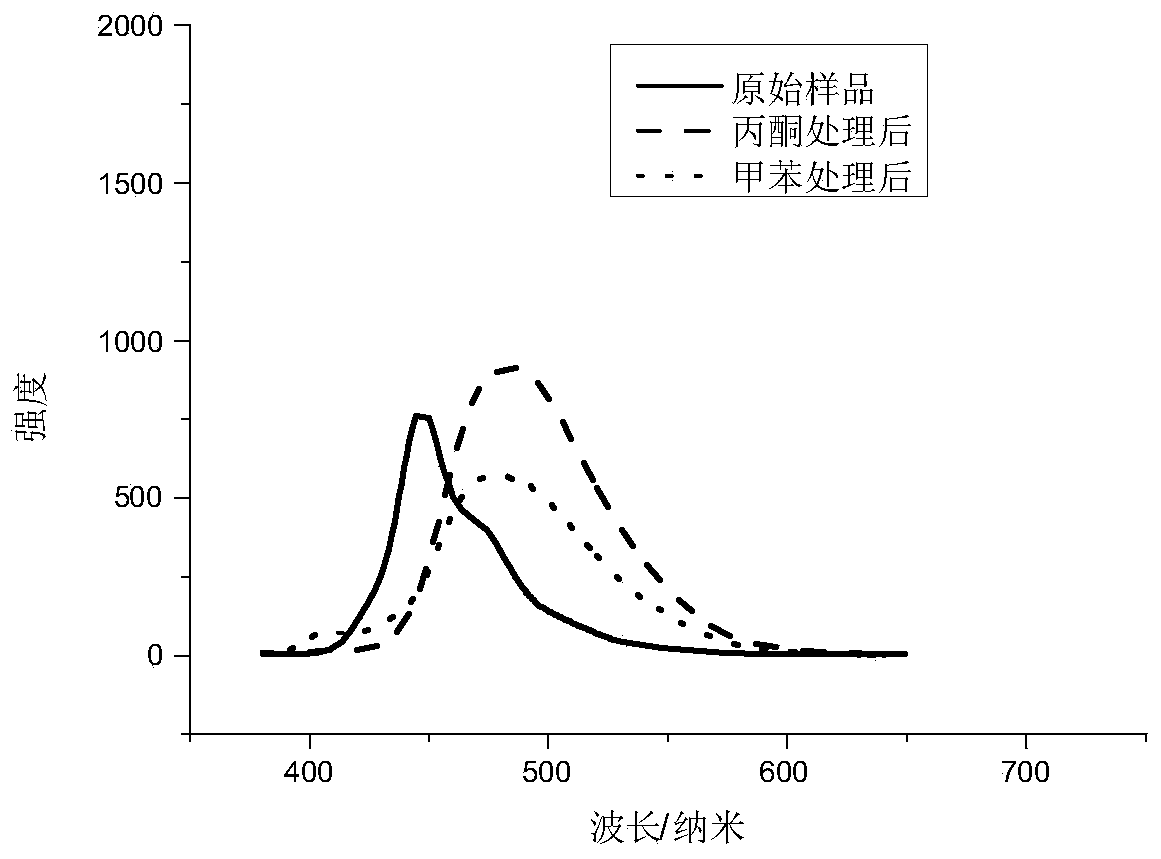
[0029] Carry out powder XRD test to the powder of product, and diffraction pattern shows that new substance is produced; Carry out single crystal X-ray diffraction analysis to single crystal product and know, (1,4'-bis[2-(o-cyanophenyl)vinyl ]benzene) and 4-bromo-2,3,5,6-tetrachlorobenzoic acid are bound together by intermolecular hydrogen bonds to form a new crystal structure;
[0030] Analyzing and measuring the fluorescence spectra of product powders exposed to different solvents s...
Embodiment 2
[0033] 1) Weigh 0.0664g of (1,4-bis-p-(cyanostyrene)-benzene) and 0.1088g of octafluoronaphthalene;
[0034] 2) Mix the two evenly, put them into a ball mill (Leicher MM200, Germany), add 30 μL of chloroform, and grind for 30 minutes at 30 r / s;
[0035] 3) Dissolve the ground powder in 10mL of chloroform, place it at room temperature, and wait for the solvent to evaporate to obtain a single crystal product.
[0036] 4) Characterize the product:
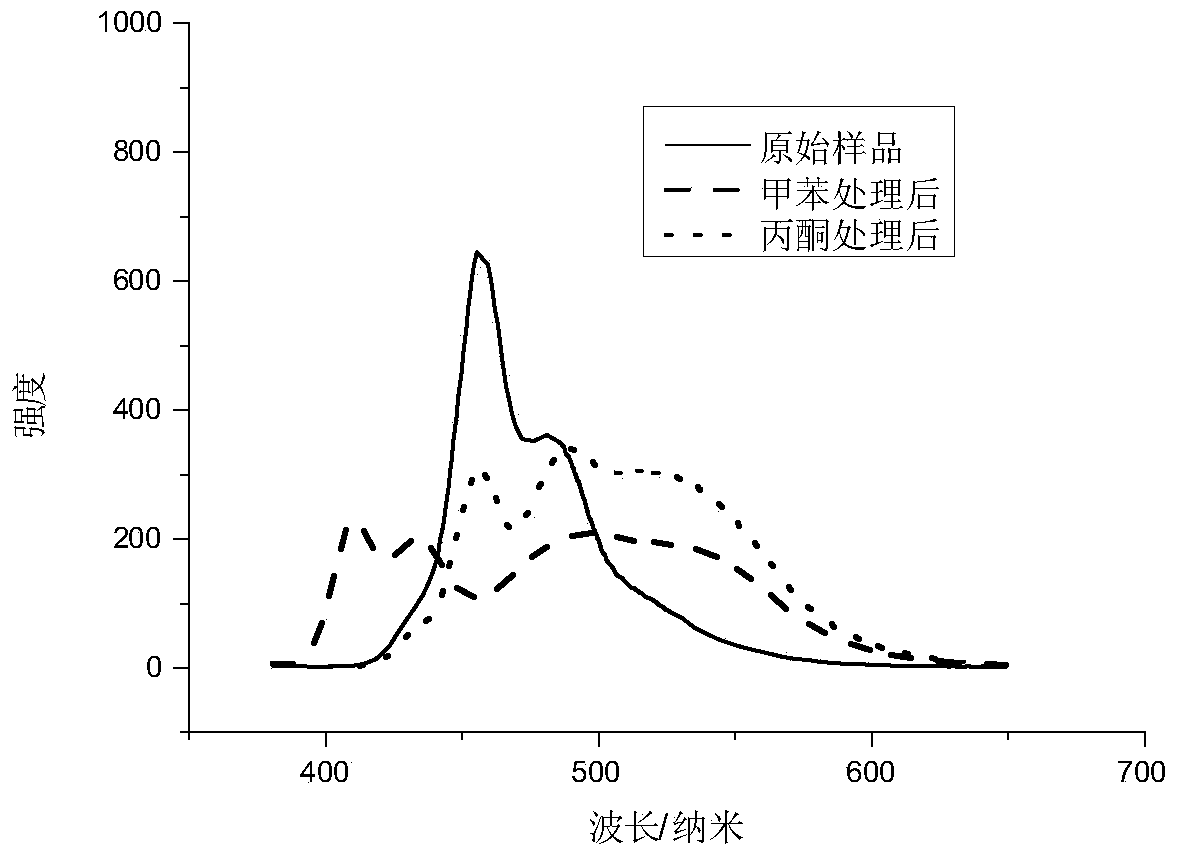
[0037] The powder of the product was tested by PXRD, and the diffraction pattern showed that new substances were produced; the single crystal X-ray diffraction analysis of the single crystal product showed that (1,4-bis-p-(cyanostyrene)-benzene) Combined with octafluoronaphthalene through intermolecular halogen bonds to form a new crystal structure;
[0038] Different solvents are added dropwise to the product powder for fluorescence spectrum measurement, and different products have specific responses to different solvents. ( ima...
Embodiment 3
[0040] 1) Weigh 0.0664g (1-o-cyano-styryl, 4-p-cyano-styryl) benzene and 0.1088g octafluoronaphthalene;
[0041] 2) Mix the two evenly, put them into a ball mill, add 3 drops of chloroform, and grind at 20r / s for 30min;
[0042] 3) Take about 0.1 g of the ground powder and dissolve it in 10 mL of chloroform, place it at room temperature, and wait for the solvent to evaporate to obtain a single crystal product.
[0043] 4) Characterize the product:
[0044] The XRD test was carried out on the powder of the product, and the diffraction pattern showed that new substances were produced; the single crystal X-ray diffraction analysis of the single crystal product showed that (1-o-cyano styryl, 4-p-cyano styryl) Benzene and octafluoronaphthalene produced co-crystals through intermolecular interactions, forming a new crystal structure;
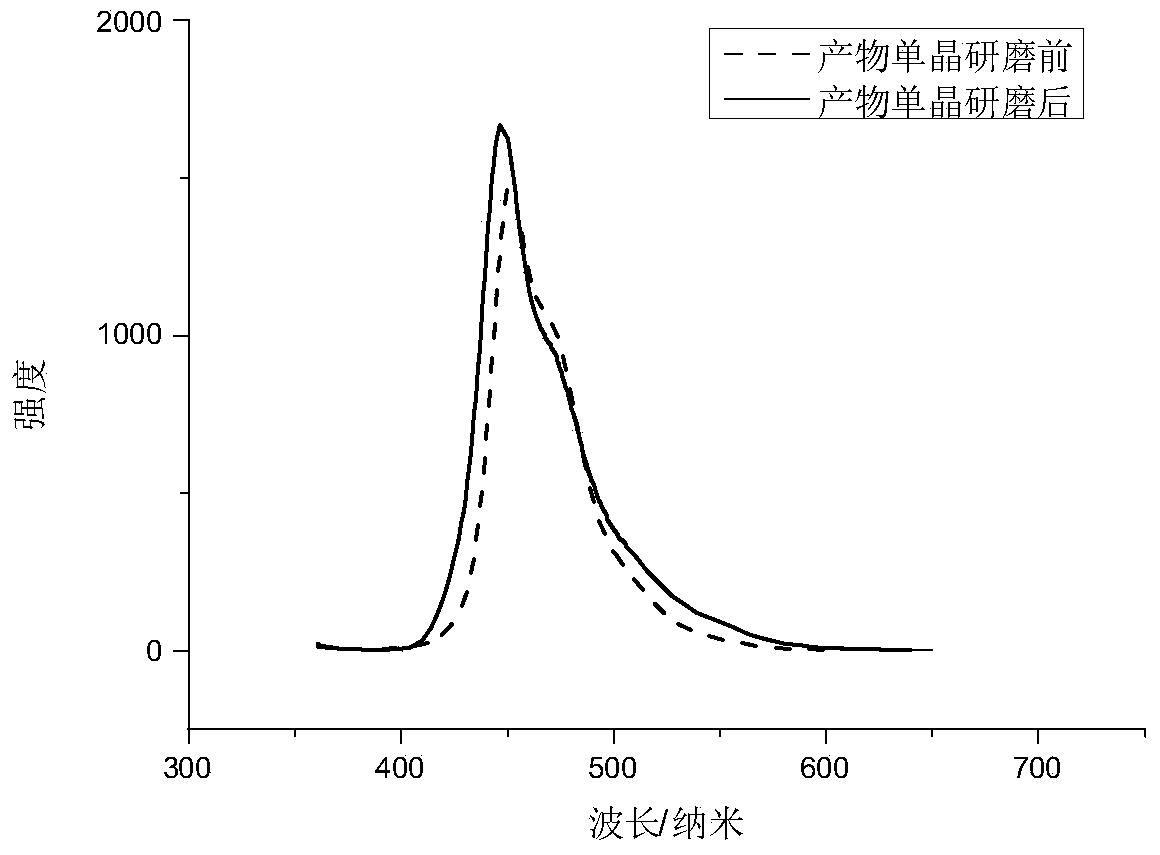
[0045]The product single crystal was placed under full-spectrum light conditions (light source: Zhongjiao Jinyuan CEL-HXB300 / UV300; 20mA), illumina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com