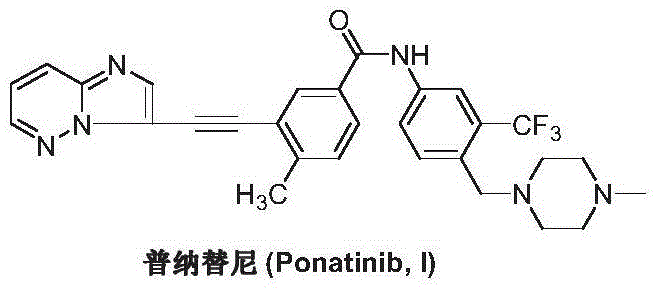
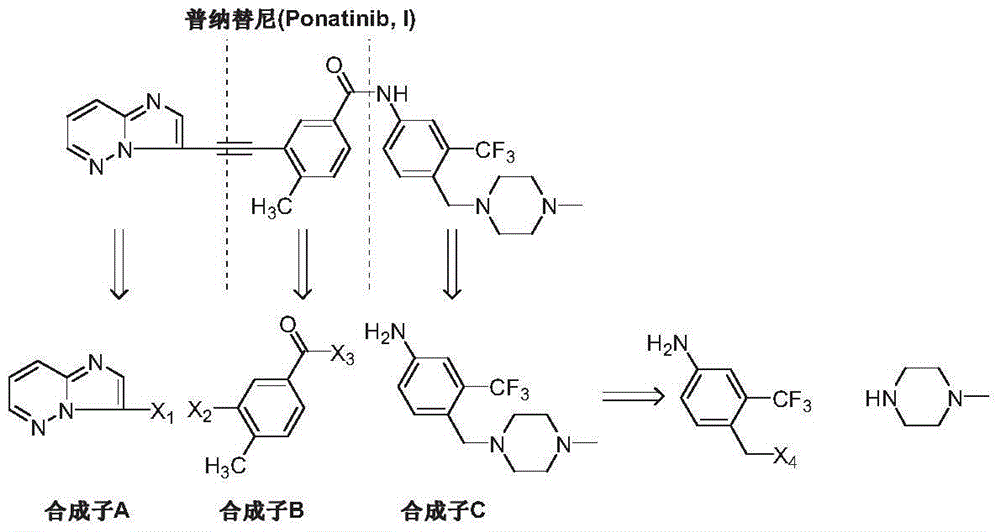
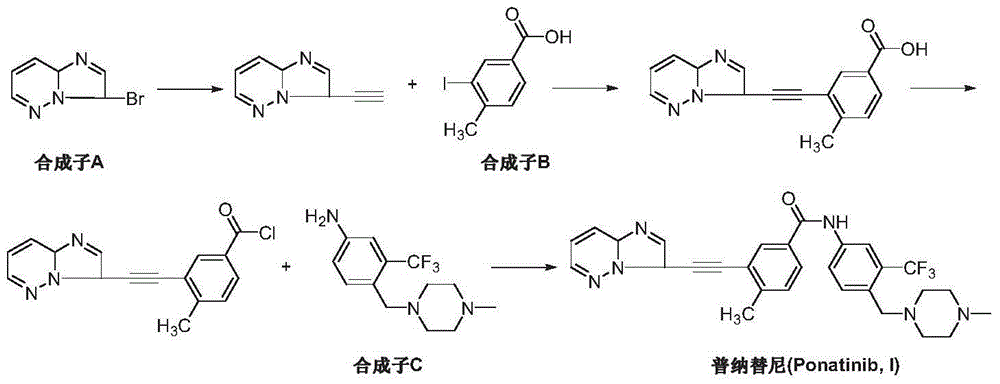
The preparation method of ponatinib
A technology of ponatinib and imidazolium, applied in the field of preparation of ponatinib, can solve the problems of high preparation cost, unfavorable industrialization, long reaction time and the like, and achieve the effects of promoting development, easy availability of raw materials, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 4-methylbenzoic acid (III) (13.6 g, 0.1 mol) and 200 mL of 1,2-dichloroethane into a dry three-necked reaction flask, add aluminum trichloride (26.6 g, 0.2 mol), and imidazo[1,2-b]pyridazine-3-carbonyl chloride (II) (20.1 g, 0.11 mol) in 100 mL of 1,2-dichloroethane was added dropwise. The temperature was raised to 50-60° C. for 5 hours, and TLC detected that the reaction was complete. Cool down to room temperature, add 1M dilute hydrochloric acid to quench the reaction, filter, and wash the filtrate with saturated sodium bicarbonate solution, saturated brine and pure water successively, and dry over anhydrous magnesium sulfate. Filtration, the filtrate was concentrated, and the resulting crude product was recrystallized from toluene and n-hexane (2:1) to obtain an off-white solid 3-(imidazo[1,2-b]pyridazin-3-yl)-(4-methylbenzoic acid -3-yl)methanone (IV) 22.1g, yield 78.1%.
Embodiment 2
[0033] Add 3-(imidazo[1,2-b]pyridazin-3-yl)-(4-methylbenzoic acid-3-yl)methanone (IV) (2.83g, 10mol ) and 50 mL of tetrahydrofuran, cooled to -78°C with acetone on dry ice. Under the protection of nitrogen, add lithium hexamethyldisilazide (2.0 g, 12 mmol) and diethyl dichloromethylphosphonate (2.4 g, 11 mmol) in 25 mL of tetrahydrofuran dropwise, and keep the reaction at -78 ° C After 30 minutes, the temperature was slowly raised to 0°C, and the reaction was continued for 1 hour. Cool down to -78°C again, add n-butyllithium (14mL, 22mmol) dropwise under stirring, raise the temperature to 0°C within 30 minutes, continue to stir and react for 40 minutes, and detect the reaction by TLC. Add 20 mL of water to quench the reaction. It was extracted three times with ether, and the organic phases were combined, washed with water, dilute hydrochloric acid and water successively, and dried over anhydrous magnesium sulfate. Concentrate under reduced pressure, add deionized water to t...
Embodiment 3
[0035] Add 3-(imidazo[1,2-b]pyridazin-3-yl)-(4-methylbenzoic acid-3-yl)methanone (IV) (2.83g, 10mol ) and 50 mL of toluene, cooled to -78°C with acetone on dry ice. Under the protection of nitrogen, add lithium hexamethyldisilazide (2.0 g, 12 mmol) and diethyl dichloromethylphosphonate (2.4 g, 11 mmol) in 25 mL of tetrahydrofuran dropwise, and keep the reaction at -78 ° C After 30 minutes, the temperature was slowly raised to 0°C, and the reaction was continued for 1 hour. Add 0.25 g of polyethylene glycol (PEG-400) and potassium hydroxide (1.23 g, 22 mmol), react at room temperature for 12 hours, and detect the reaction by TLC. Add 20 mL of water to quench the reaction. It was extracted three times with ether, and the organic phases were combined, washed with water, dilute hydrochloric acid and water successively, and dried over anhydrous magnesium sulfate. Concentrate under reduced pressure, add deionized water to the residue, stir and crystallize at room temperature. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com