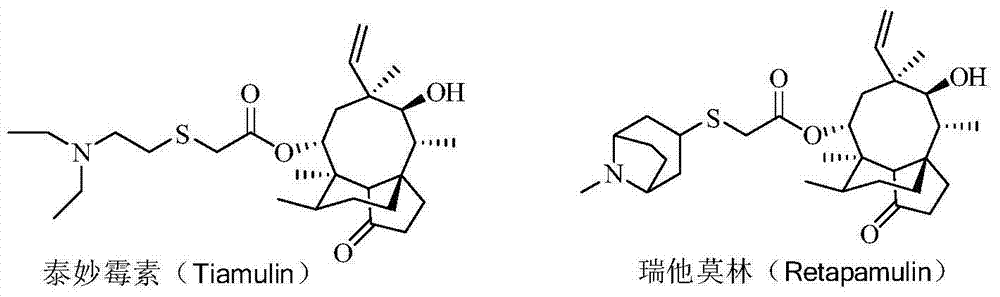
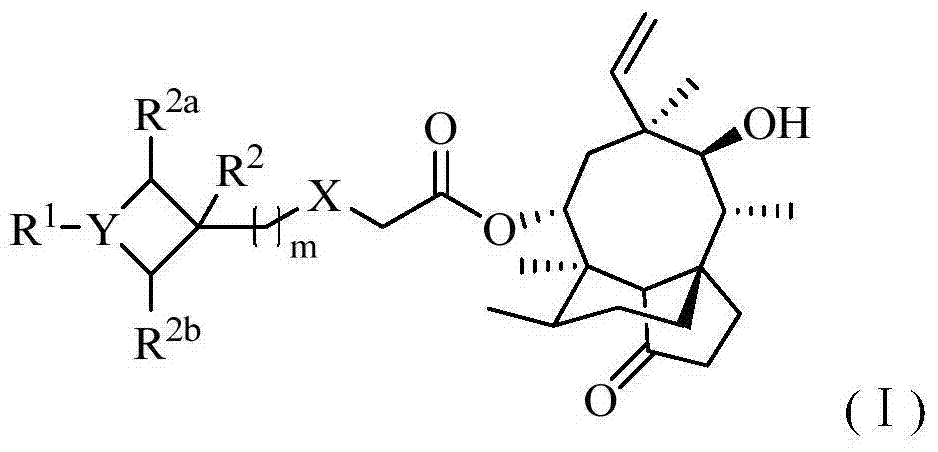
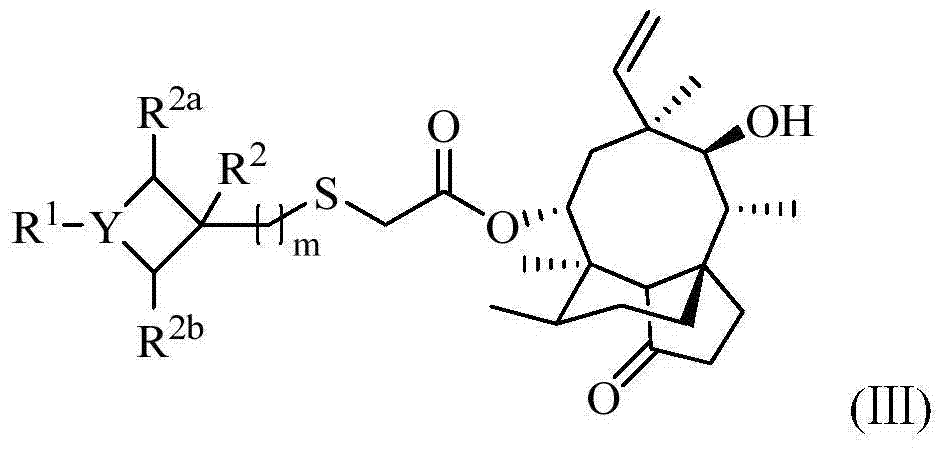
Pleuromutilin antibiotics
A technology of alkyl and compound, applied in anti-inflammatory agent, antibacterial drug, sulfide preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0279] The preparation of embodiment 14-O-(p-toluenesulfonylacetyl) tiamulin (intermediate M1)
[0280]
[0281] Tiamulin (SM1, 37.8g, 0.1mol) was dissolved in 60ml of dichloromethane, and triethylamine (15.15g, 0.15mol) was added under nitrogen protection. Stir for 30 minutes, cool down to -15°C, and add dropwise TosCl (13.75 g, 0.12 mol) into a dichloromethane solution. The mixture was stirred for 2 hours, and 1.15 L of water was added slowly. The organic phase was separated, dried, and spin-dried to obtain the product intermediate M1 (44g, 96%).
[0282] With reference to the above preparation method, the following compounds can also be prepared:
[0283]
Embodiment 1
[0284] The preparation of embodiment 1 compound 1
[0285]
[0286] (1) Preparation of intermediate 664602
[0287]
[0288] Dissolve SM2 (10.9g, 0.1mol) in 200mL MeOH, stir and react at -10°C for 30mins, then add TEA (30.3g, 0.3mol). After reacting for 2h, add (Boc) 2 O (21.8 g, 0.1 mol). The reaction was stirred overnight at -10°C. After completion of the reaction, concentrate in vacuo. Add water and DCM (200ml), stir for 10min. Separate the organic phase, wash with saturated brine, anhydrous Na 2 SO 4 Dry, filter, and concentrate to give crude intermediate 664602 (17 g, 98%).
[0289] (2) Preparation of intermediate 664603
[0290]
[0291] A solution of oxalyl chloride (6.36mmol) in DCM (25ml) was cooled to -78°C, and DMSO (12.7mmol), intermediate 664602 (1g, 5.78mmol) and TEA (3.25ml) were added sequentially. After rising to room temperature, stir the reaction for 15h. Dilute with water, stir and separate the layers, separate the organic phase, wash twice...
Embodiment 2
[0321] The preparation of embodiment 2 compound 2
[0322]
[0323] With reference to the method of Example 1, the synthetic route is as follows:
[0324]
[0325] The preparation of compound 2 was 0.8g, and the yield was 60%.
[0326] Mass Spectrum (m / e): 518(M+H) +
[0327] 1 H NMR (400MHz, MeOD, δ ppm )δ:6.34(m,1H),5.75(m,1H),5.15-5.19(m,2H),4.66(m,1H),4.43(m,1H),4.06-4.22(m,1H),3.84 -4.01(m,2H),3.78(m,1H),3.51(m,1H),3.33(m,3H),2.07-2.42(m,6H),1.83(m,1H),1.53-1.75(m ,3H),1.45(s,4H),1.34(m,3H),1.17(m,4H),1.07(m,6H),0.95(m,3H),0.73(m,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com