Use of the antibody I-3859 for the detection and diagnosis of cancer
An antibody and CDR-H3 technology, applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-tumor drugs, etc., can solve the problems of tumor growth and angiogenesis without effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0242] Example 1: Production of anti-CXCR4I-3859 monoclonal antibody (Mab)
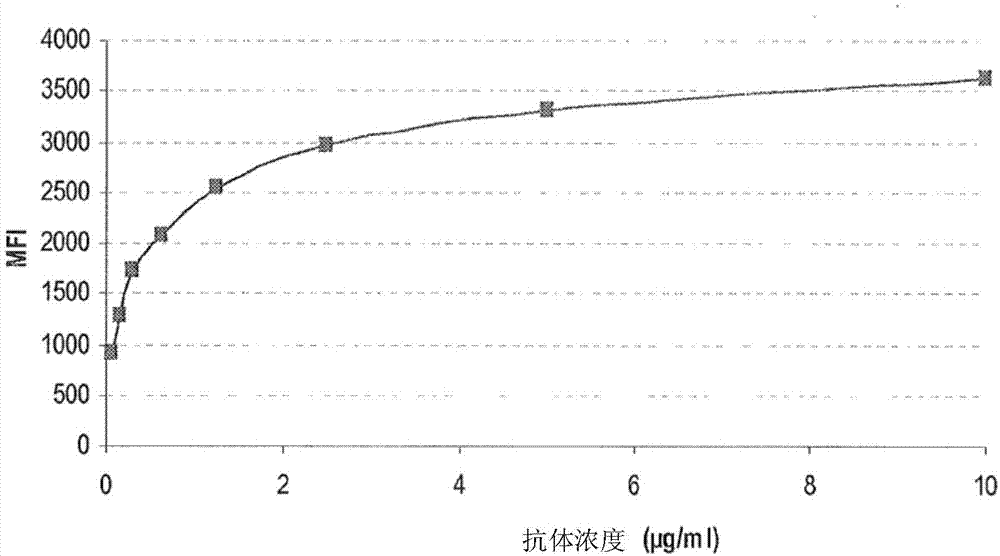
[0243] To generate monoclonal antibodies against CXCR4, Balb / c mice were immunized with recombinant NIH3T3-CXCR4 cells and / or peptides corresponding to the extracellular N-terminus and loop of CXCR4. At the time of the first immunization, immunize 6-16 week-old mice once subcutaneously (s.c.) with the antigen in complete Freund's adjuvant, followed by 2 to 6 immunizations with the antigen s.c. in incomplete Freund's adjuvant. Second-rate. The immune response was monitored by retro-orbital bleeds. Sera were screened by ELISA (as described below), and mice with higher titers of anti-CXCR4 antibodies were used for fusions. Mice were boosted intravenously with antigen two days before sacrifice and splenectomy.
[0244] -ELISA
[0245] To select mice producing anti-CXCR4 antibodies, sera from immunized mice were tested by ELISA. Briefly, microtiter plates were coated with purified [1-41]N-terminal pep...
Embodiment 2
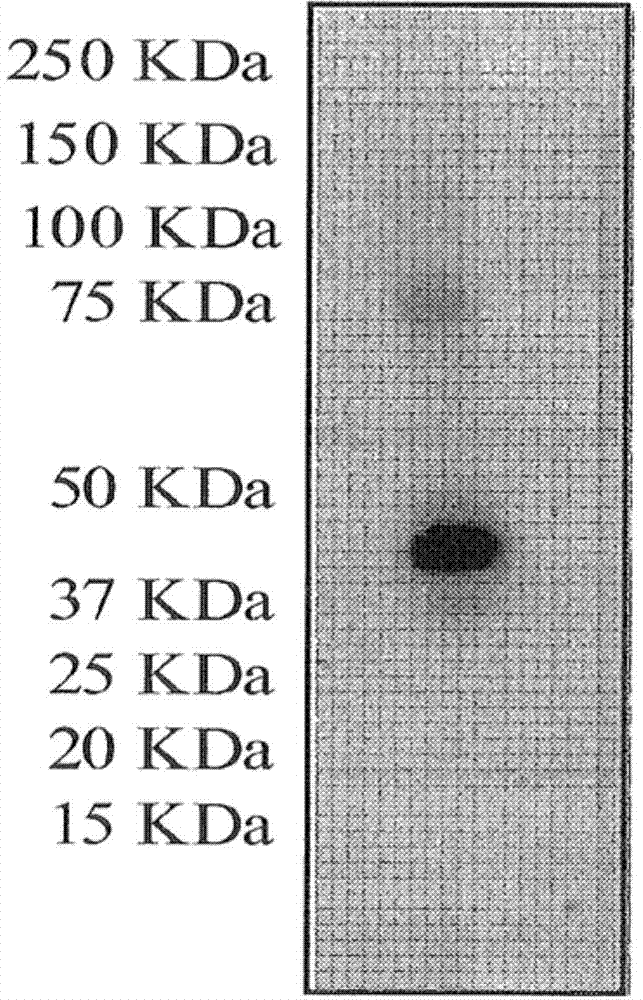
[0248] Example 2: 1-3859 Mab Immunoprecipitation of Both CXCR4 Monomers and Dimers
[0249] The NIH3T3-CXCR4 cell pellet was washed with 20 mM Tris HCl containing 100 mM (NH4)2SO4, pH 8.5, and then resuspended in lysis buffer (20 mM TrisHCl, pH 8.5, containing 100 mM glycerol, 1% CHAPSO, and 10 μL / mL protease inhibitor mixture). Cells were disrupted using a Potter Elvehjem homogenizer. The solubilized membrane was collected by centrifugation at 105,000 g for 1 h at +4°C, subsequently incubated with I3859 mAb-conjugated Sepharose 4B beads overnight at +4°C, the mixture was poured into a glass column, and washed with lysis buffer. Proteins captured by I3859 mAb were eluted and analyzed by western blotting with anti-CXCR4 mAb as primary antibody. Fragments of interest were pooled, concentrated and used for WB analysis preparative SDS-PAGE resolution (4-12% Bis-Tris gel).
[0250] After silver staining, the band of interest was excised from the gel and submitted to in-gel diges...
Embodiment 3
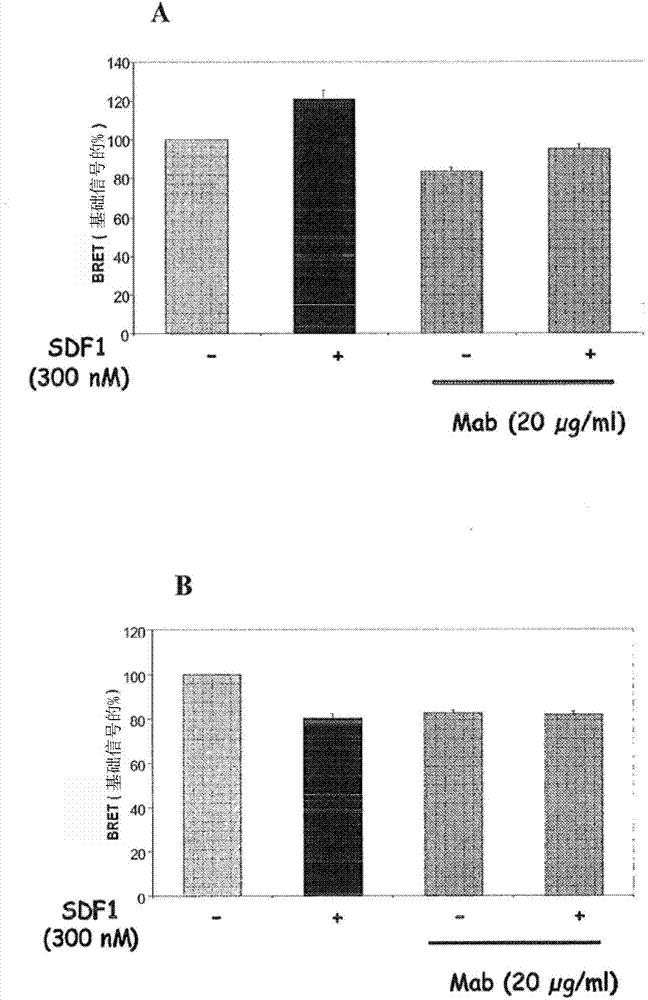
[0256] Example 3: 1-3859 Mab Modulates Both CXCR4 Homodimers and CXCR4 / CXCR2 Heterodimers Assayed by BRET
[0257] This functional analysis allows assessment of conformational changes induced by binding of SDF-1 and / or I-3859 mAbs to the CXCR4 receptor at the level of CXCR4 homodimer and CXCR2 / CXCR4 heterodimer formation.
[0258] Expression vectors were constructed as fusion proteins with the corresponding dyes (Renilla reniformis luciferase (Rluc) and yellow fluorescent protein (YFP)) for each investigated interaction partner using traditional molecular biology techniques . Two days before the BRET experiment, HEK293 cells were transiently transfected with an expression vector encoding the corresponding BRET partner: [CXCR4 / Rluc+CXCR4 / YFP for studying CXCR4 homodimerization ], and [CXCR4-Rluc+CXCR2-YFP] for studying CXCR4 and CXCR2 heterodimerization. One day later, cells were dispersed in complete medium [DMEM supplemented with 10% FBS] on poly-lysine pre-coated white 96M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com