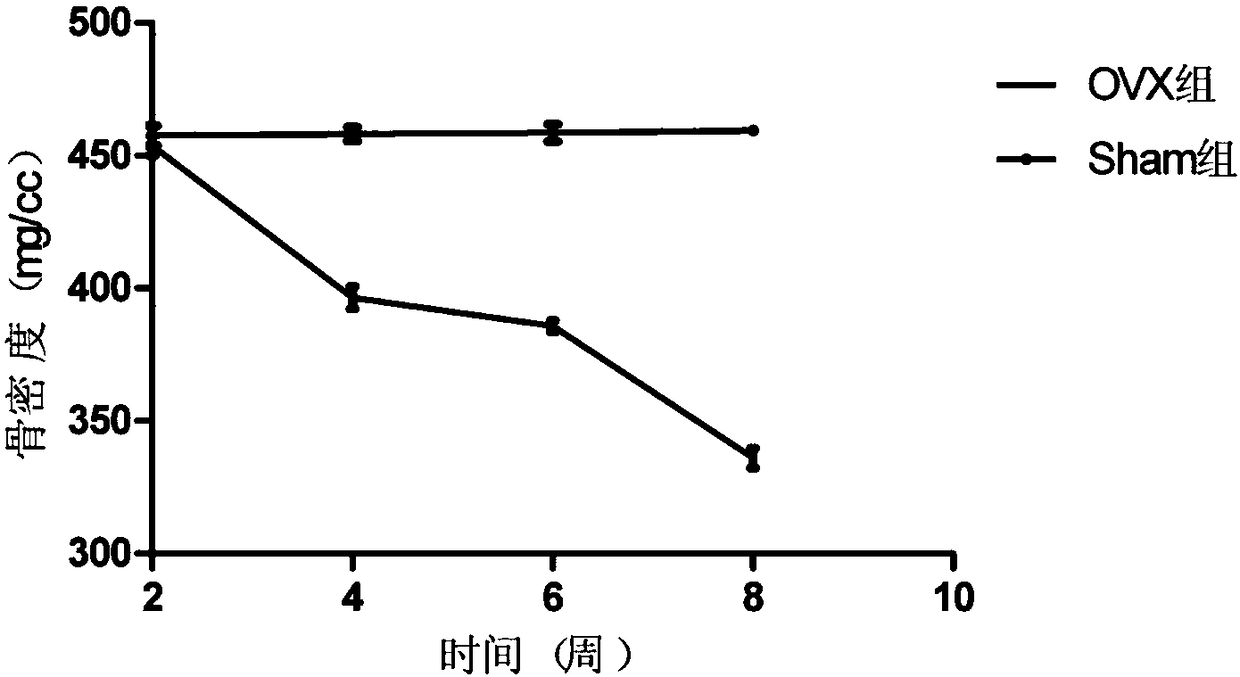
Patents
Literature
39 results about "Antibodies i" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
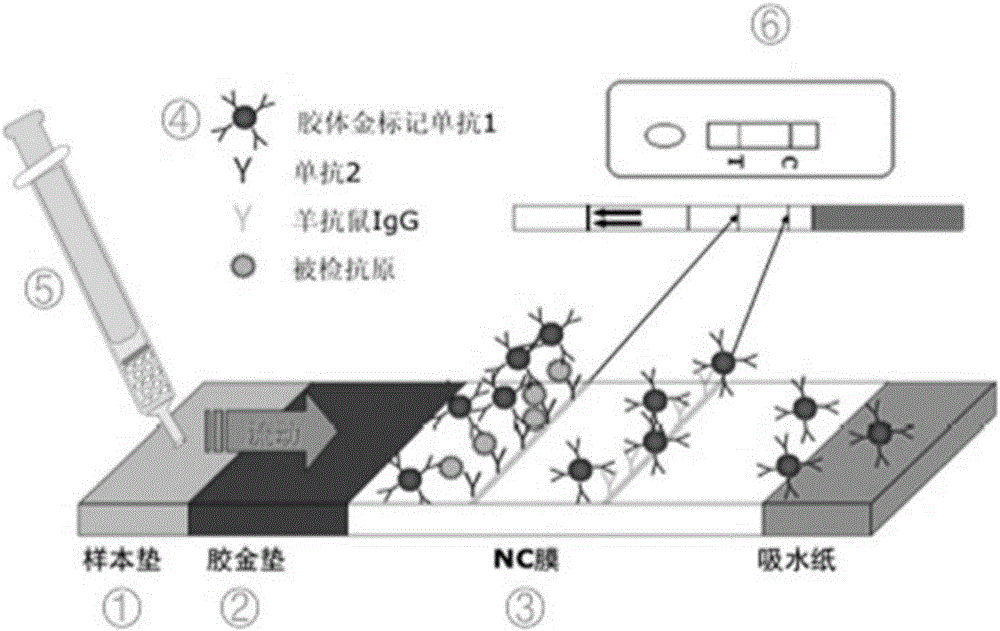
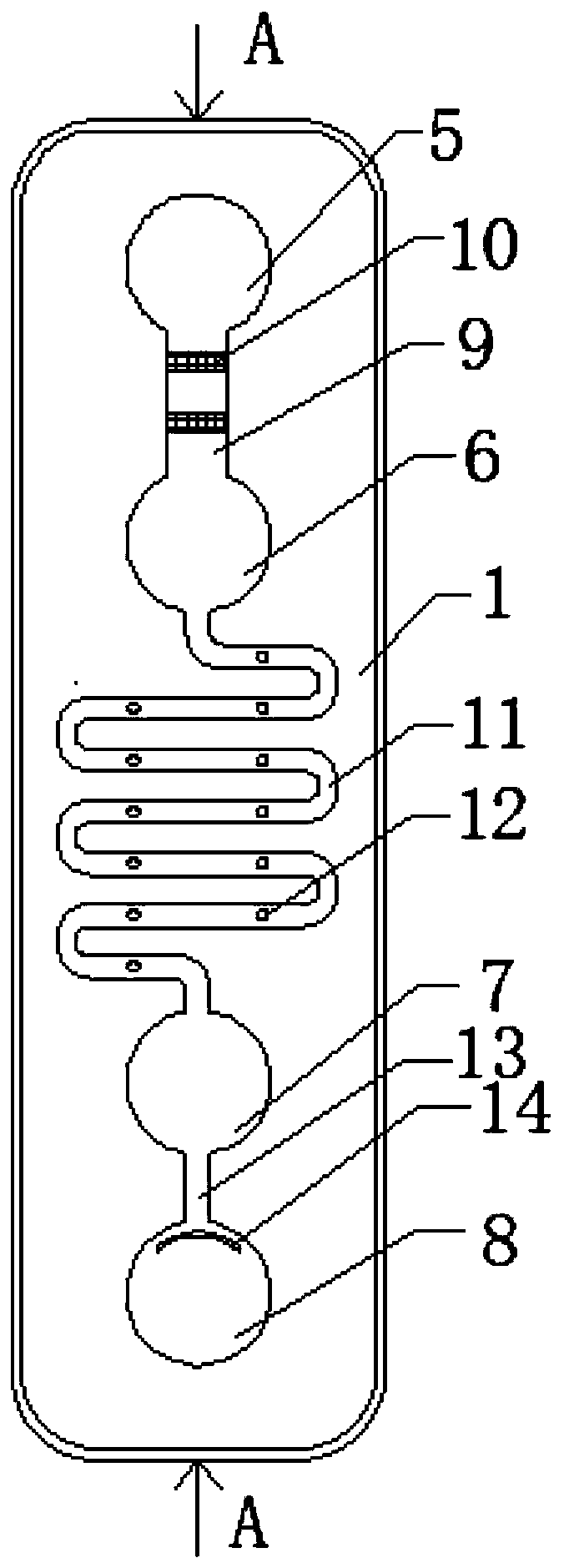
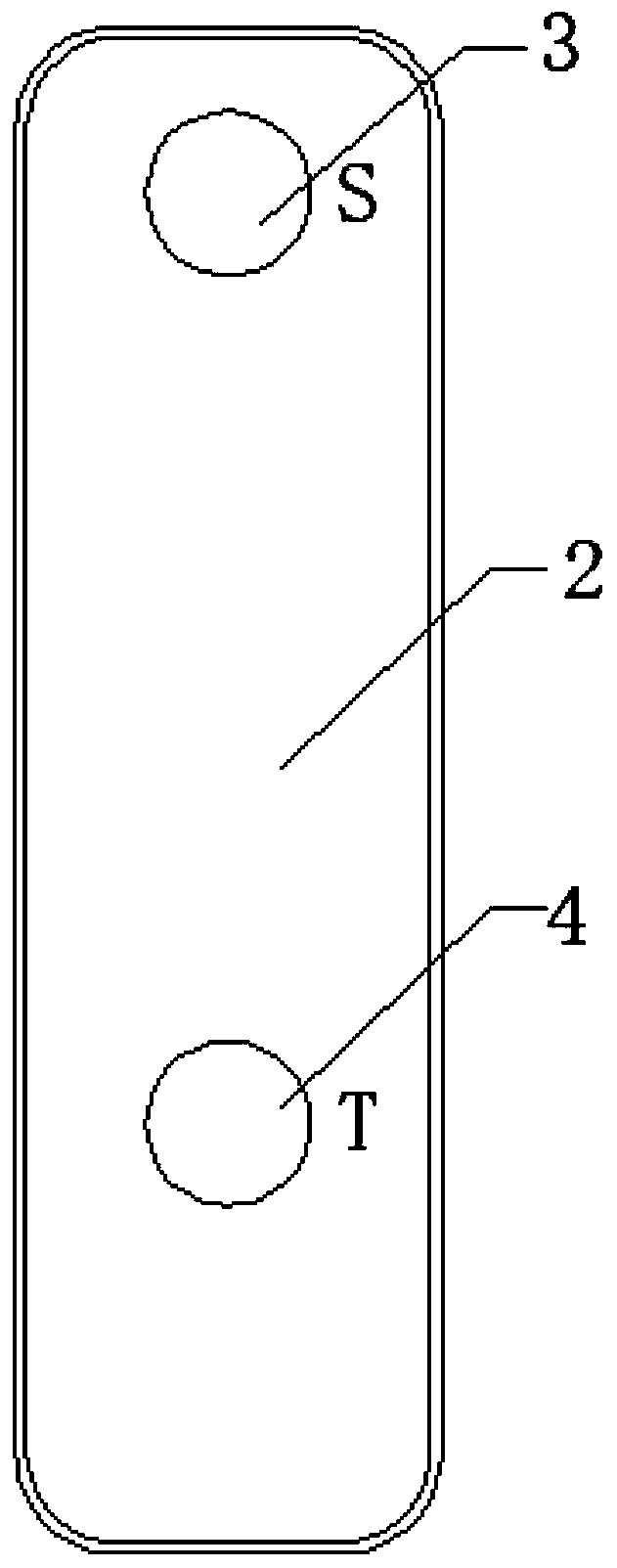
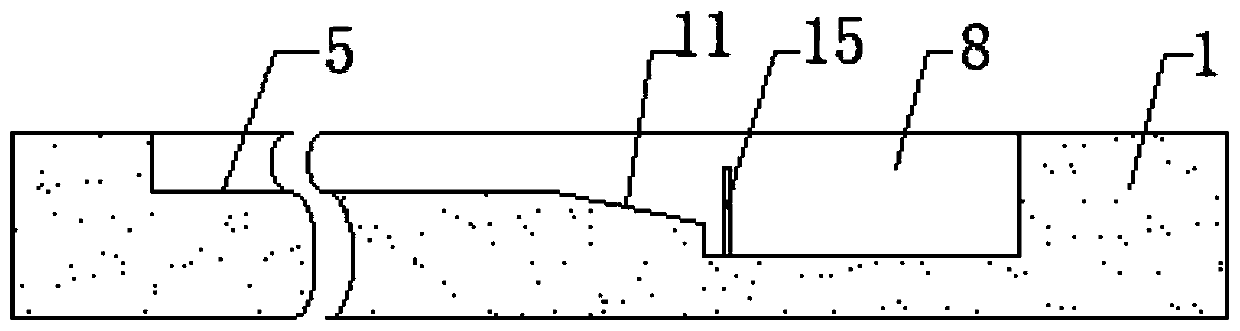
Apparatus for simultaneously detecting 10 kinds of food microorganism and screening diagnosis method thereof
InactiveCN101470115AWith quantitative samplingWith scientific samplingMaterial analysisMicroorganismMonoclonal antibody
The invention relates to a device for checking 10 food microbes synchronously and a screen diagnosis method, which adopts check test paper and a sample cup to process one-time synchronous check on 10 food microbes. The invention is characterized in that an immunity method is selected to select two matched monoclonal antibodies for different antigenic determinants of one strain as a food microbe antibody I and a food microbe antibody II; the invention utilizes prepared matched monoclonal antibodies and utilizes color particle immunity chromatography method to prepare test paper; the 10 food microbe test paper are arranged in one sample cup, and when adds a liquid sample, the one displays two read lines is considered as the existence of one microbe. The invention combines general food microbe agents together, to detect 10 microbes for one sample, thus being convenient for screening and on-time diagnosis.
Owner:万积成
Quantitative determination RBP4 kit by chemiluminescence magnetic enzymoimmune method
ActiveCN101452001AExtended storage timeStable LuminescenceChemiluminescene/bioluminescenceBiological testingImmunocompetenceMagnetic bead
The invention relates to a medical testing kit for performing quantitative detection on human serum RBP4 using chemiluminescence magnetic-enzyme immunotherapy. The kit is composed of four reagent parts: specificity mouse anti-human RBP4 custodite immunomagnetic beads, enzyme labelling specificity mouse anti-human RBP4 antibody II, chemiluminescence substrate, corresponding titer and quality control liquid. The using method of the kit comprises: using bead particulates as solid phase carrier, combining specificity mouse anti-human RBP4 antibody I on the surface, forming RBP4 specificity immunocompetence beads, capturing antigen RBP4 to be detected in the enzyme labelling specificity mouse anti-human RBP4 antibody II, forming double antibody sandwich composite on the surface of the beads, wherein enzyme marked on the composite reacts with corresponding irradiance substrate in the reaction system to form stable luminous signals, thereby reaching quantitative detection and analysis on RBP4 through strength of the detection light signals. The invention has the advantages of high sensitivity, high specificity, simple and fast operation.
Owner:WUHAN EASYDIAGNOSIS BIOMEDICINE
Detection kit of helicobacter pylori emulsion method and preparation process thereof
The invention relates to the field of the emulsion method immune chromatography, and particularly relates to a detection kit of a helicobacter pylori emulsion method. The detection kit of the helicobacter pylori emulsion method comprises a reagent strip, wherein the reagent strip comprises a substrate, filter sample paper, a sensitized emulsion polyester film, an immune cellulose nitrate film and absorbent paper, wherein the filter sample paper, the sensitized emulsion polyester film, the immune cellulose nitrate film, and the absorbent paper are orderly connected end to end, and are fixed on the substrate, the immune cellulose nitrate film is covered with helicobacter pylori resisting urea enzyme antibodies I marked by colorful emulsion particles, and the immune cellulose nitrate film is provided with a detection line coated with helicobacter pylori resisting urea enzyme antibodies II and a quality control line coated by goat anti-rabbit serum IgG. The detection kit of the helicobacter pylori emulsion method, which is provided by the invention, has the advantages of convenience in detection, stability in detection results and cost conservation.
Owner:SHANGHAI CHEMTRON BIOTECH
Surface enhanced Raman scattering immunochromatography test paper strip and preparation method and application
ActiveCN105259158ANo pollution in the processOperational securityRaman scatteringBiological testingLap jointTest strips
The invention discloses a surface enhanced Raman scattering immunochromatography test paper strip and a preparation method and application. The test paper strip comprises a bottom liner, a sample absorbing cushion, a combination cushion, a chromatography membrane and a water absorbing cushion. The sample absorbing cushion, the combination cushion, the chromatography membrane and the water absorbing cushion are closely connected in sequence and are attached to the bottom liner, wherein gold-kernel and silver-shell nanoflower marked antibodies I adhere to the combination cushion, a detection line is arranged on the chromatography membrane, and antibodies II or antigens A adhere to the detection line. According to the surface enhanced Raman scattering immunochromatography test paper strip and the preparation method and application, due to the fact that the gold-kernel and silver-shell nanoflower marked antibody I are dispersed on the combination cushion, the antigens A or the antibodies II linearly adhere to the chromatography membrane, and the detection line is formed; the sample absorbing cushion, the combination cushion, the chromatography membrane and the water absorbing cushion are sequentially connected to the bottom liner in a lap joint mode; the surface enhanced Raman scattering immunochromatography test paper strip is obtained. The surface enhanced Raman scattering immunochromatography test paper strip has the advantages of being safe to operate, easy and convenient to use, low in cost, fast to use, high in sensitivity and the like and is wide in application range.
Owner:广州华澳生物科技有限公司
Antigen detection method and application thereof
InactiveCN101566626AEasy to store and transportHigh sensitivityMicrobiological testing/measurementMaterial analysisPsa antigenMicrosphere
The invention relates to an antigen detection method and application thereof. The method converts detection of an antigen signal into detection of a nucleic acid barcode label, and comprises the following operation steps: (1) labeling modified nano magnetic microspheres by using an antibody I of an antigen to be detected to prepare immune magnetic microspheres, and labeling nano-gold sol by using an antibody II of the antigen to be detected and a nucleic acid barcode fragment at the same time to prepare a double-labeled nano-gold probe; (2) fully combining the immune magnetic microspheres, the nano-gold probe and the antigen to be detected, removing combined aggregate, and leaving supernatant fluid for detection; and (3) detecting the nucleic acid barcode label in the supernatant fluid. The antigen detection method can be applied to the detection of clinical PSA antigen. The method has the advantages of simple operation, high sensitivity, good specificity, easy automation and wide application prospect.
Owner:SHENZHEN PEOPLES HOSPITAL
New method for sensitivity of supermolecular self-assembling mediated net-shaped nano-gold enhanced immunochromatographic test strip
ActiveCN108459159AHigh detection sensitivityStrong specificityMaterial analysisBovine serum albuminPorphyrin structure
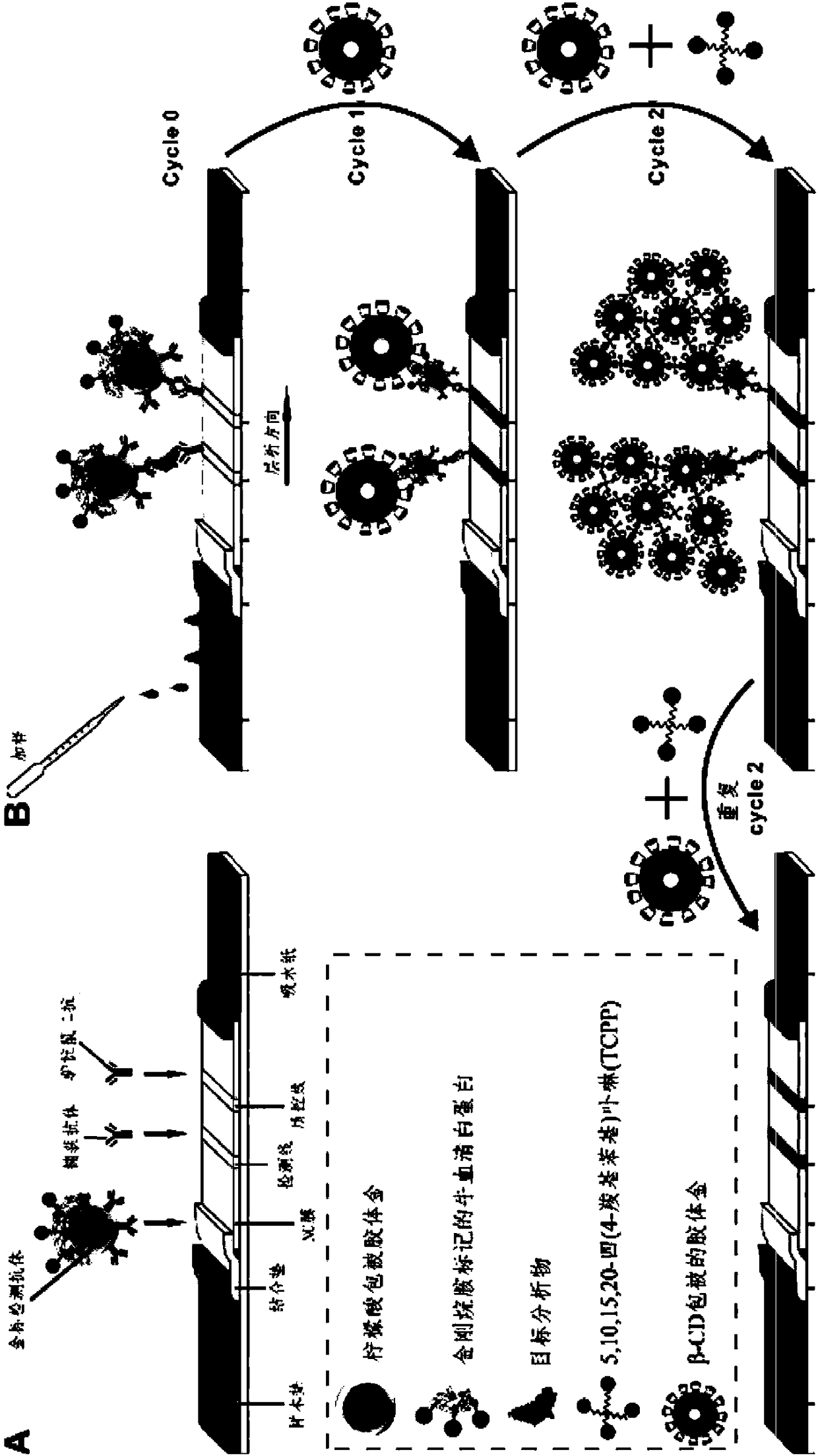
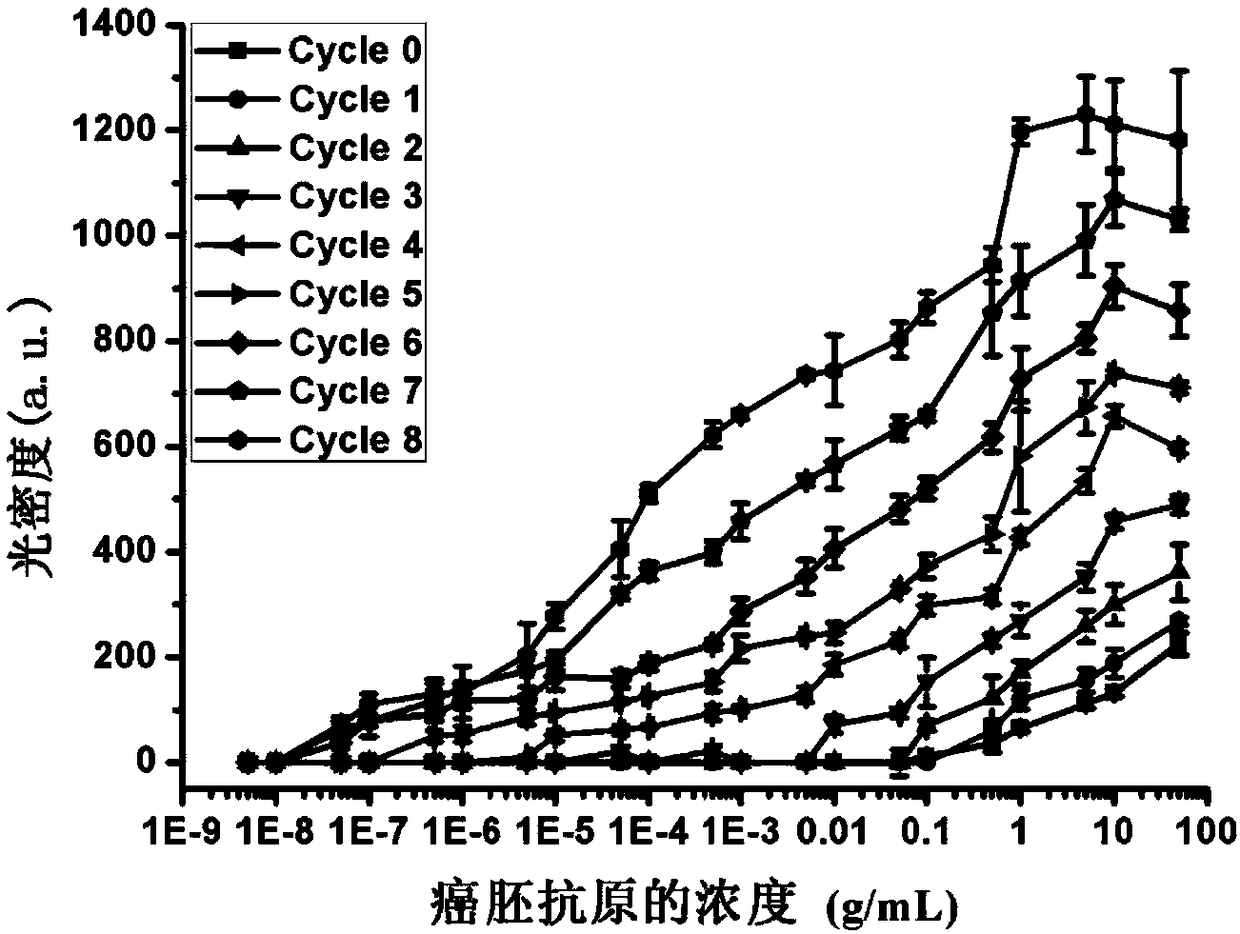
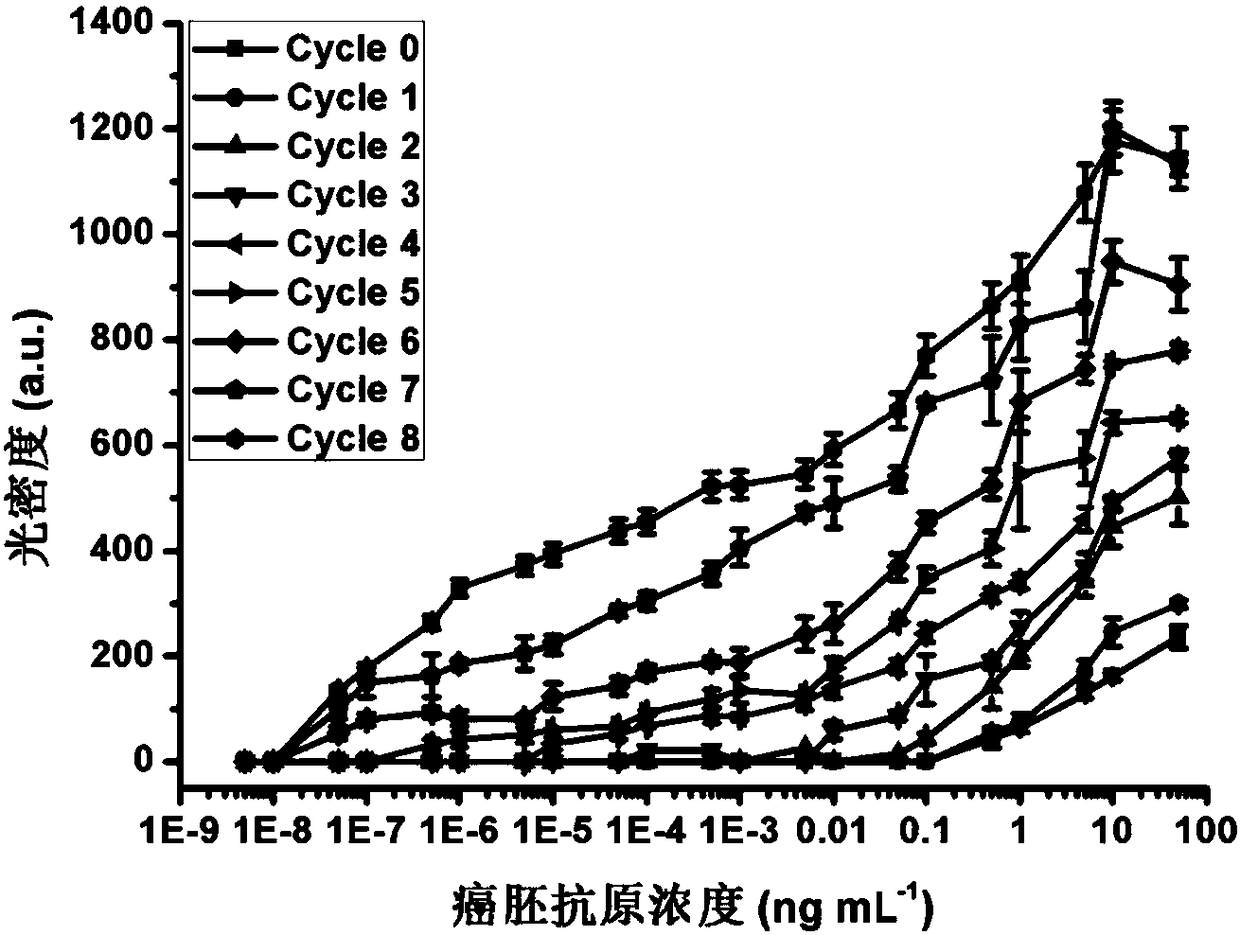
The invention provides a method for amplifying colloidal gold immunochromatographic colorimetric signal intensity. The method comprises the following steps: closing a gold-labelled antibody by using amantadine marked bovine serum albumin to obtain a gold-labelled antibody I; synthesizing colloidal gold nanoparticles by using beta-cyclodextrin to obtain a beta-cyclodextrin coated colloidal gold solution; after adding a to-be-tested sample into a system which executes double-antibody sandwich colloidal gold immunochromatography by using the gold-labelled antibody I, adding the beta-cyclodextrincoated colloidal gold solution; and adding a mixed solution of 5,10,15,20-tetra(4-carboxyl phenyl)porphyrin and the beta-cyclodextrin coated colloidal gold solution into the system repeatedly for manytimes. According to the method, net-shaped nano-gold is formed by utilizing supermolecular self-assembling principle mediation and circular signal amplification is realized, so that the developing intensity of the god nanoparticles on a detection line and a quality control line is greatly improved and the aim of performing on-site rapid detection on a target detection object with ultralow concentration can be fulfilled. The invention also provides a high-sensitivity kit applied to colloidal gold immunochromatography.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
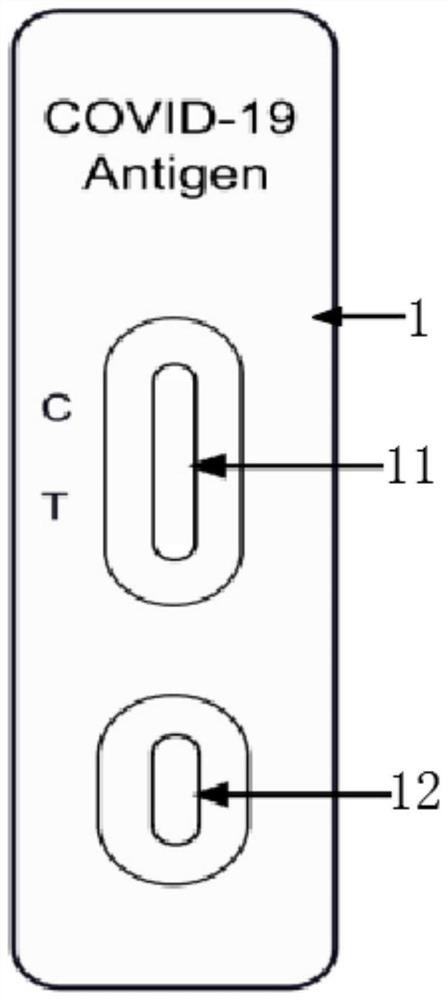
Novel coronavirus (COVID-19) antigen detection kit and detection method thereof
PendingCN112129937AStrong specificityThe detection process is fastBiological testingImmunoassaysProtein.monoclonalEpidemic spread
The invention relates to the technical field of novel coronavirus detection, and discloses a novel coronavirus (COVID-19) antigen detection kit. The novel coronavirus (COVID-19) antigen detection kitcomprises a cuboid paper box package, wherein a detection card, a sterile swab, a sample extraction solution, a sample extraction tube, a suction head and a specification are arranged in the cuboid paper box package; the detection card comprises a card shell and a test strip; the test strip comprises a sample pad, a marking pad, an NC film, absorbent paper and a PVC bottom plate; and the marking pad is provided with a colloidal gold-marked murine N protein monoclonal antibody I. The detection kit disclosed by the invention is high in specificity, high in detection speed and simple and convenient to operate, does not need special equipment or professional operation, can be applied to preliminary screening of various places such as communities, primary hospitals, airports, customs and even families, can judge results within several minutes, and provides a simpler, more convenient and faster field detection means for suspected patient investigation and asymptomatic infected person screening, thereby preventing epidemic spread as soon as possible.
Owner:深圳容金科技有限公司
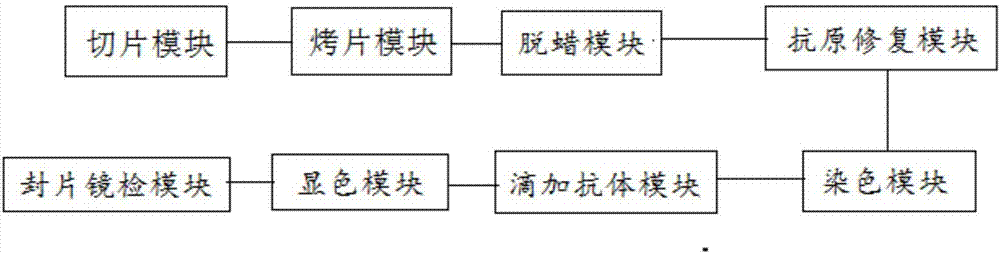
Pathological specimen slice immunocytochemistry operation method and pathological specimen slice immunocytochemistry operation system
The invention discloses a pathological specimen slice immunocytochemistry operation method and a pathological specimen slice immunocytochemistry operation system. The pathological specimen slice immunocytochemistry operation method comprises: slicing pathological specimen paraffin, baking the pathological specimen paraffin slice, removing the paraffin from the pathological specimen paraffin slice, carrying out antigen repair on the pathological specimen slice, staining the pathological specimen slice, adding antibodies to the pathological specimen slice in a dropwise manner, carrying out color developing on the pathological specimen slice, counterstaining the pathological specimen slice, sealing the pathological specimen slice, carrying out microscopic examination, and other steps. According to the present invention, staining is firstly performed with hematoxylin and then the antibodies I and II are added in the dropwise manner, such that the color developing of the antibodies can be easily achieved, the size and the position of the tissue can be easily grasped by sequentially carrying out the staining and the antibody adding in the dropwise manner, the position deviation during the antibody adding in the dropwise manner is reduced, the normative experiment operation can be easily performed, the strong practical value can be provided, the actual waste can be effectively avoided, and the error of the experiment data can be substantially reduced.
Owner:CHANGSHA KINGMED MEDICAL DIAGNOSTICS INST
Use of the antibody I-3859 for the detection and diagnosis of cancer
InactiveCN103717620AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsCXCR4Diagnosis cancer
Owner:PIERRE FABRE MEDICAMENT SAS
Test strip for rapidly detecting plasmodia based on colloidal gold immunochromatographic assay, as well as antibodies and cell lines thereof
InactiveCN102229913AEasy to operateRapid diagnosisMicroorganism based processesTissue cultureCellulosePolyester
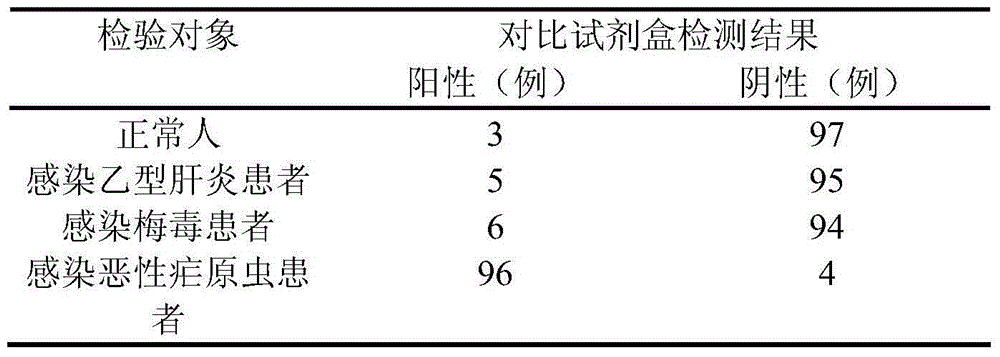
The invention discloses a test strip for rapidly detecting plasmodia based on colloidal gold immunochromatographic assay, as well as antibodies and cell lines thereof. The test strip comprises a basal plate, a sample pad, a polyester cellulose pad, a nitrocellulose membrane and a water absorbent pad, wherein the sample pad, the polyester cellulose pad, the nitrocellulose membrane and the water absorbent pad are partially superposed in sequence along the length direction of the basal plate and fixed on the basal plate; a colloidal gold-antibody I (H12) conjugate coating is sprayed on the polyester cellulose pad; a first test line, a second test line and a quality control line are distributed on the nitrocellulose membrane; the first test line is formed by performing spray-coating on an antibody II (A5) on the nitrocellulose membrane; the second test line is formed by performing spray-coating on an antibody IV (F6) on the nitrocellulose membrane; and the quality control line is formed by performing spray-coating on an goat anti-mouse IgG1 antibody on the nitrocellulose membrane. The test strip provided by the invention is easy to operate, rapid, sensitive and specific, and is applied to the rapid and early-stage diagnosis of malaria.
Owner:SOUTHERN MEDICAL UNIVERSITY
Immunohistochemical operation method
ActiveCN105973681AGood colorEasy to operateMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationCooking & bakingRoom temperature
The invention discloses an immunohistochemical operation method. The immunohistochemical operation method comprises the following steps of: (1) slicing and baking slices; (2) dewaxing and hydrating; (3) repairing an antigen; (4) sealing endogenous catalase; (5) dyeing; (6) dropwise adding an antibody I; (7) dropwise adding an antibody II; (8) developing, namely developing by adopting a DAB developing agent at a room temperature; and (9) sealing the slices. According to the technical scheme disclosed by the invention, a conventional operation step of dropwise adding the antibody I and the antibody II before dyeing by haematoxylin, the antibody can be easily developed after the antibody I and the antibody II are dropwise added. The immunohistochemical operation method is simple in operation steps; only an operation sequence of existing operation steps, adopted reagents and operation time are changed and the change of an existing operation effect can be realized; the antibodies are dropped after dyeing so that the size and position of tissues can be conveniently grasped. The operation steps are simple and certain technical detail problems in experiment check analysis can be effectively solved; standard operation of an experiment are easy to realize, wastes of experiment reagents can be avoided and certain errors in the experiment are also reduced as much as possible.
Owner:SICHUAN KINGMED DIAGNOSTICS CENT
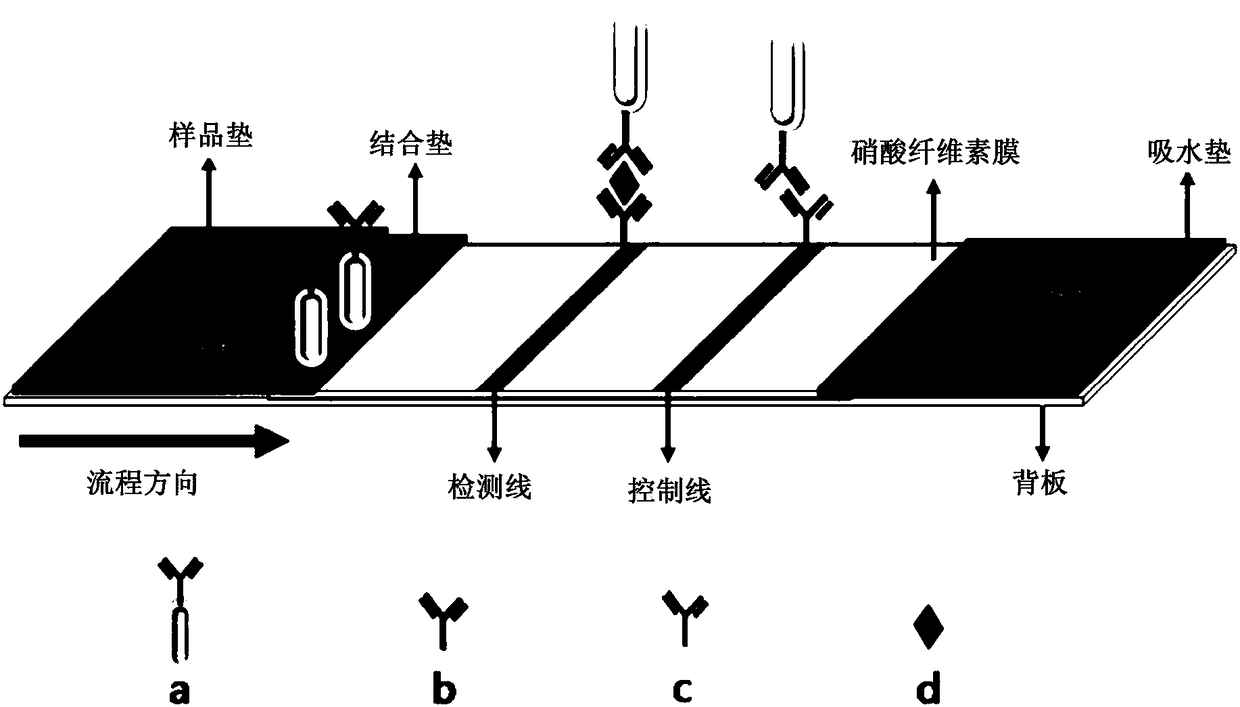
Immunochromatographic test strip and preparation method thereof
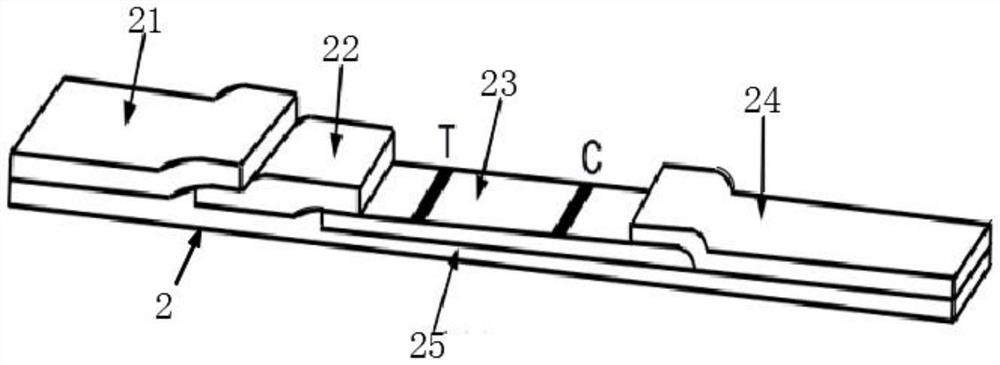
The invention relates to an immunochromatographic test strip and a preparation method thereof. The immunochromatographic test strip comprises a sample pad, a combination pad and a reaction film whichare arranged on a substrate, the combination pad is coated with a first marker and a second marker; wherein the first marker is a compound in which a target antibody I is coupled to a labeled microsphere I, the second marker is a compound in which the target antibody I and a quality control substance I are jointly coupled to a labeled microsphere II, the detection line is coated with a target antibody II, and the quality control line is coated with a quality control substance II capable of being specifically combined with the quality control substance I. The test strip provided by the invention adopts a new quality control system, not only eliminates the interference of a T-line antibody to a C line and the interference of a common blocking agent and a common interfering substance, but also changes the signals of the T line and the C line along with the change of the concentration of a target object, effectively improves the detection precision, and widens the linear detection range.
Owner:东莞市东阳光诊断产品有限公司
Nano-gold test paper for detecting major outer membrane protein antigen of chlamydia trachomatis as well as preparation method and application thereof
InactiveCN102411055AHigh sensitivityEasy to storeMaterial analysisNitrocelluloseChlamydia trachomatis Antigen
The invention relates to test paper for detecting chlamydia trachomatis infection, and the test paper comprises a baseplate, a sample pad, a conjugate pad, a nitrocellulose membrane and an absorption pad, wherein a detection line and a quality control line are arranged on the nitrocellulose membrane, the detection line on the nitrocellulose membrane is coated by mouse anti-human monoclonal protein antibody IgG (antibody II) of major outer membrane protein antigen of chlamydia trachomatis in the concentration of greater than or equal to 1mg / mL, and the quality control line is coated by goat anti-mouse IgG polyclonal antibody in the concentration of greater than or equal to 1.5mg / mL; the conjugate pad is coated by mouse anti-human monoclonal protein antibody IgG (antibody I) of the MOMP (major outer membrane protein) antigen of the chlamydia trachomatis, which is labeled by nano-gold and is in the concentration of not less than 6 mu g / mL; the sensitivity of detecting the MOMP of the chlamydia trachomatis of the test paper is 0.1ng / ml, the detection value range is 0-200ng / ml and the specificity is 98.5%; and the detection can be completed within 5 minutes.
Owner:ARMY MEDICAL UNIV
Food-borne pathogenic bacteria quick detection method based on Gamma-Fe2O3@Au nano particle indirect enrichment and immunomagnetic separation
A food-borne pathogenic bacteria quick detection method based on Gamma-Fe2O3@Au nano particle indirect enrichment and immunomagnetic separation, and belongs to the technical field of quick pathogen detection for food security. On dependence of a method for detecting pathogen in a food liquid sample, the specificity of an antibody I is combined with the target bacteria, the Gamma-Fe2O3@Au composite nano particle is utilized to prepare immuomagnetic beads of an antibody II to enrich target strains marked by the antibody I, the nano magnetic beads of the target bacteria are captured through separation, and then subjected to nitration to be converted into ferrous ions, and the ferrous ions are detected to indirectly detect whether the sample contains target pathogen. Under a certain condition, the quantity of the ferrous ions and the target bacteria show linear relation, so that the target bacteria can be quantificationally detected within a certain range. The food-borne pathogenic bacteria quick detection method can be used for quick detection of harmful pathogen in the food sample, and can be used for quick screening of large quantities of samples to be detected.
Owner:NANCHANG UNIV
Colloidal gold test strip capable of simultaneously detecting waterfowl source parvoviruses and preparation method
InactiveCN106645727AReduce false positivesImprove featuresBiological material analysisNitrocelluloseQuality control
The invention relates to the field of animal medicine, and discloses a colloidal gold test strip capable of simultaneously detecting waterfowl source parvoviruses and a preparation method. The colloidal gold test strip comprises a base plate, a sample pad, a gold mark pad, a nitrocellulose membrane and an absorbent pad, the gold mark pad is coated with colloidal gold mark protein with gosling plague virus monoclonal antibodies I, detection lines and quality control lines are sequentially arranged on the nitrocellulose membrane along flowing directions of samples, the detection lines of the nitrocellulose membrane are coated with gosling plague virus monoclonal antibodies II, and the quality control lines of the nitrocellulose membrane are coated with goat-anti-mouse IgG (intravenous gamma globulin) antibodies. The colloidal gold test strip can simultaneously detect goose parvoviruses, Muscovy duck parvoviruses and novel Muscovy duck parvoviruses, false positive of reaction is reduced to a great extent, and specificity and sensitivity of detection are improved.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +1
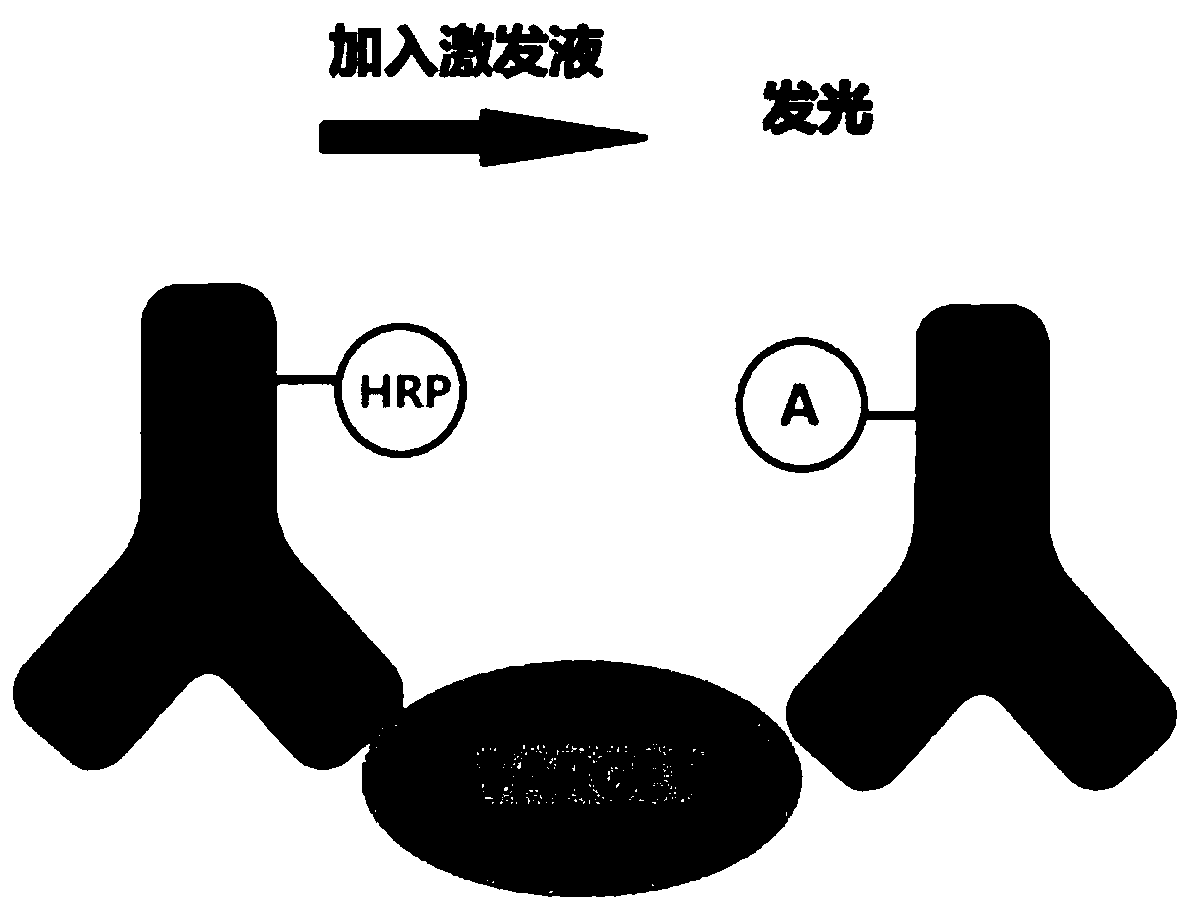
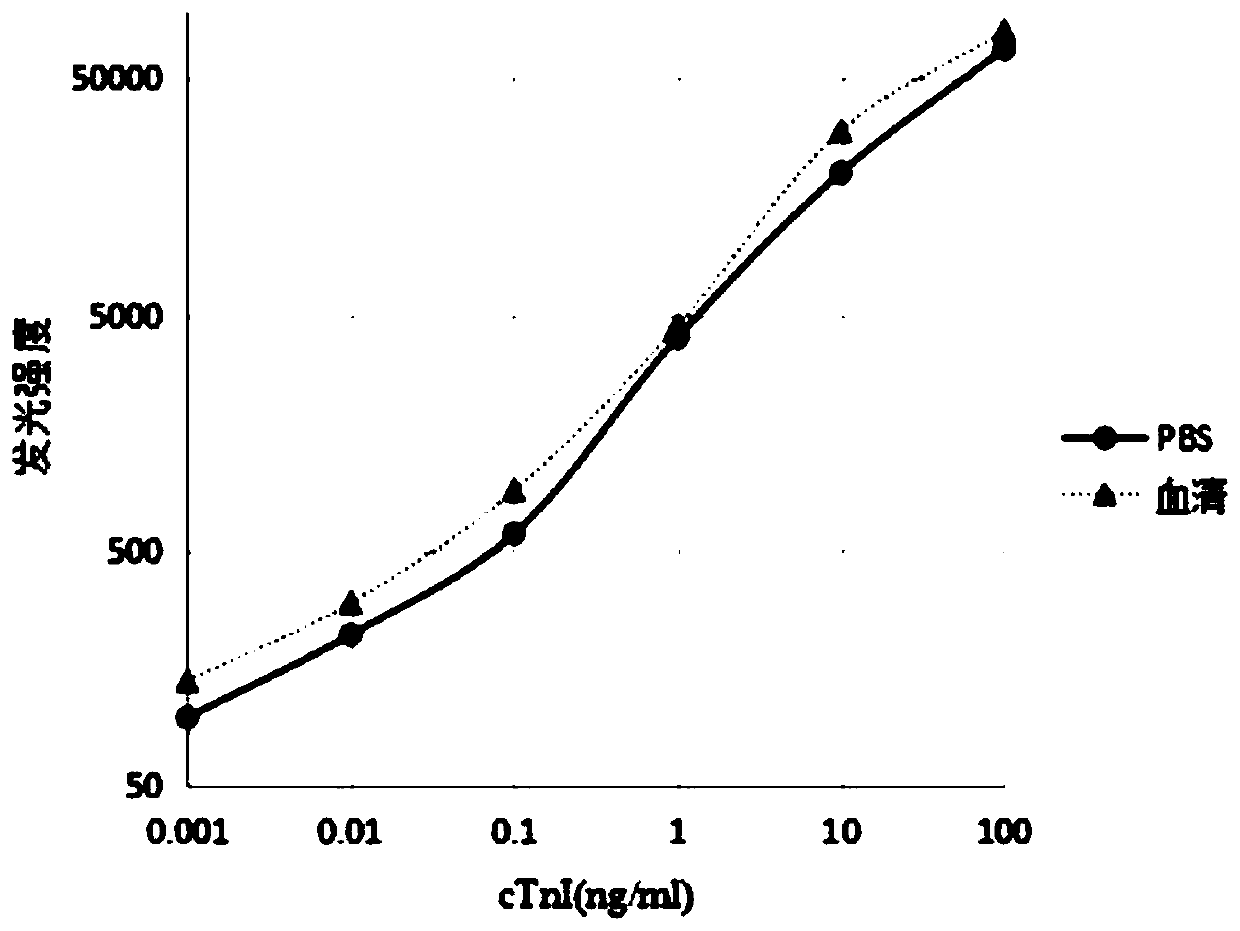
Method for detecting cardiac troponin I/T through flash type homogeneous chemiluminescence technology
PendingCN110031635AAdequate immune responseNo wash operationChemiluminescene/bioluminescenceDisease diagnosisChemiluminescenceTroponin I
The invention discloses a method for detecting cardiac troponin I / T through a flash type homogeneous chemiluminescence technology. Serum is used as a sample to be detected. The method comprises the steps of: coupling an antibody I to a luminous substrate, so that an antibody I-A is obtained; coupling an antibody II to horse radish peroxidise, so that an antibody II-HRP is obtained; designing two detection systems, adding exciting fluid after incubating the detection system, and instantly detecting a light emitting signal; taking a cTnI / cTnT sample after gradient dilution as a sample, and then,performing detection, so that a formula that the light emitting signal corresponds to the cardiac troponin I / T concentration is obtained; and then, performing detection by taking the sample to be detected as the sample, so that the cardiac troponin I / T concentration in the sample to be detected is finally obtained. The method for detecting the cardiac troponin I / T has the characteristics of beingrapid and high in sensitivity.
Owner:杭州普鲁米生物科技有限公司
Kit for early prediction of osteoporosis
ActiveCN105759053AIt has the function of risk predictionEasy to operateDisease diagnosisBiological testingBone densitySerum samples
The invention provides a kit for early prediction of osteoporosis. The kit comprises a detection plate, a standard substance, interferon-gamma antibodies I and interferon-gamma antibodies II capable of emitting fluorescent light, wherein the detection plate comprises a plate body and at least two sample adding holes in the plate body; the bottoms of the sample adding holes are coated with the interferon-gamma antibodies II; the standard substance is an interferon-gamma water solution, and the concentration of the water solution is higher than or equal to 1 ng / mL. A serum sample of a to-be-tested person and the standard substance are added to the sample adding holes in the detection plate respectively, and whether the to-be-tested person has the risk of osteoporosis is judged according to the difference of fluorescence intensity. The kit is low in cost, high in accuracy and good in reliability, has a risk prediction function by comparison with traditional bone density examination and can provide a diagnosis basis for prediction of osteoporosis in clinic.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Test strip and method for detecting prostate tumor antigens
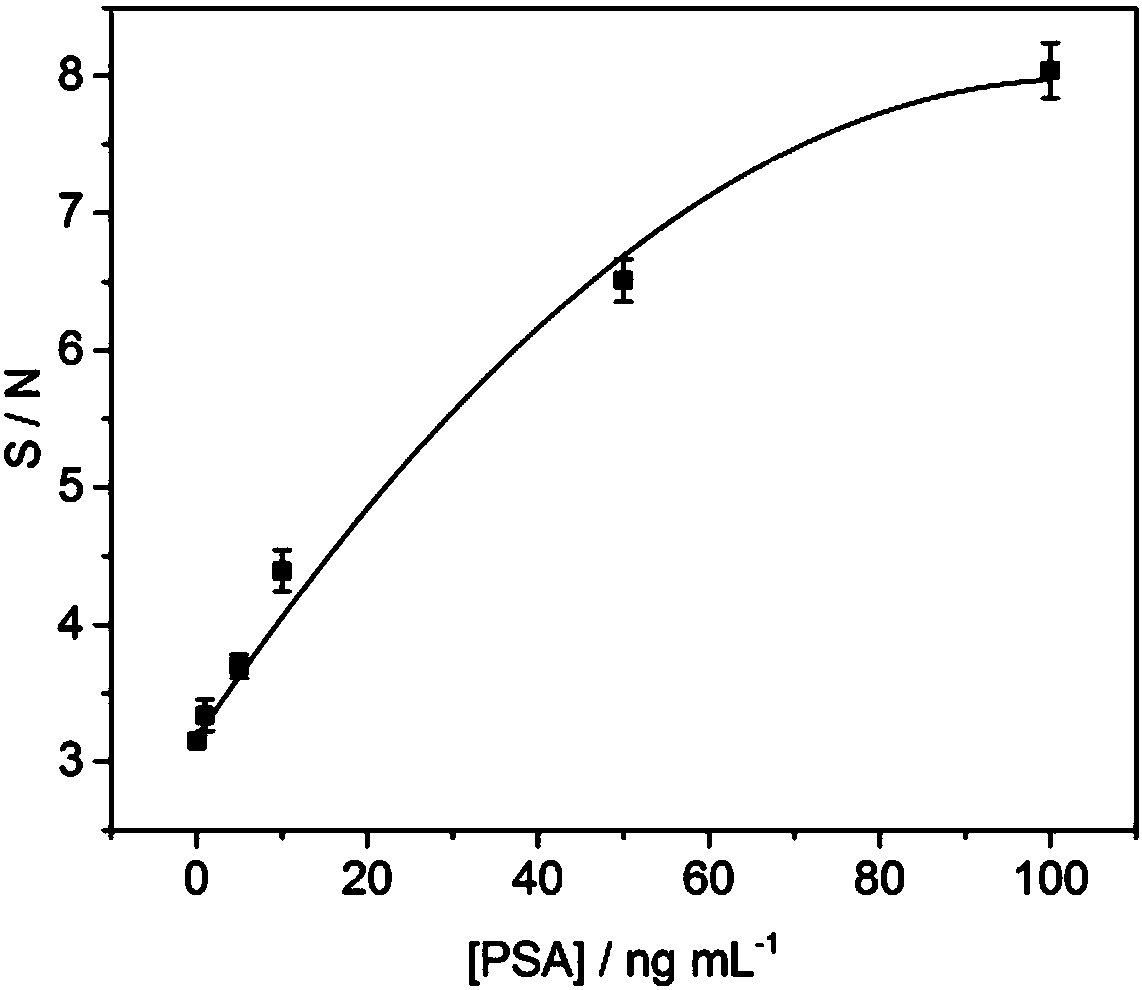
The invention provides a test strip and a method for detecting prostate tumor antigens. By the aid of the test strip and the method, the shortcomings of complicated operation and long time consuming of existing methods for detecting PSA (prostate specific antigens) and difficulty in meeting POC (point of care) quick diagnosis requirements can be overcome. PSA monoclonal antibodies II are labeled by gold nanorods by the aid of immunochromatography technologies, a nitrocellulose membrane is coated with goat anti-rat IgG and PSA monoclonal antibodies I, and the test strip for detecting the PSA isprepared by the aid of double-antibody sandwich processes. Liquid can flows through a binding pad and the nitrocellulose membrane step by step under capillary actions after PSA sample solution is added onto a sample pad of the test strip, and is bound with biological molecules, which are immobilized on the test strip, under antigen-antibody interaction, and a detection line visible to naked eyescan be generated. The test strip and the method are combined with microarray scanners, so that corresponding detection signal strength values can be obtained, and the PSA can be detected. The test strip and the method have the advantages that the method is simple and speedy, and is low in sample consumption and cost and short in detection time, the minimum concentration of the detectable PSA is 0.1 ng mL<-1>, the detection range is 0.1-100 ng mL<-1>, and clinical detection requirements can be met.
Owner:上海格荣生物科技有限公司
Novel tissue slice immunohistochemical operation method
The invention discloses a novel tissue slice immunohistochemical operation method. The novel tissue slice immunohistochemical operation method includes the steps of 1), closing endogenous catalase and soaking by 0.3% methanol-hydrogen peroxide for 30 minutes; 2), staining by hematoxylin for 2 minutes; 3), dropwise adding 40 microlitres of antibody I and standing at room temperature for 40 minutes-1 hour; 4), dropwise adding 40-50 microlitres of antibody II and standing at room temperature or 37 DEG C for 1 hour. The novel tissue slice immunohistochemical operation method has the advantages that a conventional operation step that the antibody I and the antibody II are stained by the hematoxylin before being dropwise added is changed to be beneficial to developing of the antibody I and the antibody II which are dropwise added. The novel tissue slice immunohistochemical operation method is simple in operation step and succeeds in changing existing operation effects only by making changes on operation sequences of existing operation steps, adopted reagents and operation time, and the antibodies are added dropwise after staining to facilitate confirmation of sizes and positions of tissues.
Owner:SICHUAN KINGMED DIAGNOSTICS CENT
Food-borne pathogenic bacteria quick detection method based on Fe3O4 nano particle indirect enrichment and immunomagnetic separation
A food-borne pathogenic bacteria quick detection method based on Fe3O4 nano particle indirect enrichment and immunomagnetic separation belongs to the technical field of quick pathogen detection for food security. On dependence of a method for detecting pathogen in a food liquid sample, the specificity of an antibody I is combined with the target bacteria, the Fe3O4 nano particle material is utilized to prepare immuomagnetic beads of an antibody II to enrich target strains marked by the antibody I, the nano magnetic beads of the target bacteria are captured through separation, and then subjected to nitration to be converted into ferrous ions, and the ferrous ions are detected to indirectly detect whether the sample contains target pathogen. Under a certain condition, the quantity of the ferrous ions and the target bacteria show linear relation, so that the target bacteria can be quantificationally detected within a certain range. The food-borne pathogenic bacteria quick detection method can be used for quick detection of harmful pathogen in the food sample, and can be used for quick screening of large quantities of samples to be detected.
Owner:NANCHANG UNIV
Food-borne pathogenic bacteria quick detection method based on Gamma-Fe2O3@Au nano particle indirect enrichment and immunomagnetic separation
InactiveCN103185796BQuick filterThe detection is objective and effectiveMaterial analysisBiotechnologyFood safety
A food-borne pathogenic bacteria quick detection method based on Gamma-Fe2O3@Au nano particle indirect enrichment and immunomagnetic separation, and belongs to the technical field of quick pathogen detection for food security. On dependence of a method for detecting pathogen in a food liquid sample, the specificity of an antibody I is combined with the target bacteria, the Gamma-Fe2O3@Au composite nano particle is utilized to prepare immuomagnetic beads of an antibody II to enrich target strains marked by the antibody I, the nano magnetic beads of the target bacteria are captured through separation, and then subjected to nitration to be converted into ferrous ions, and the ferrous ions are detected to indirectly detect whether the sample contains target pathogen. Under a certain condition, the quantity of the ferrous ions and the target bacteria show linear relation, so that the target bacteria can be quantificationally detected within a certain range. The food-borne pathogenic bacteria quick detection method can be used for quick detection of harmful pathogen in the food sample, and can be used for quick screening of large quantities of samples to be detected.
Owner:NANCHANG UNIV
Quantitative immune colloidal gold detection card and kit for urocystatin C, urine microalbumin and urine creatinine
PendingCN110988365AImprove the detection rateImprove detection accuracyDisease diagnosisBiological testingElevated creatinineColloidal au
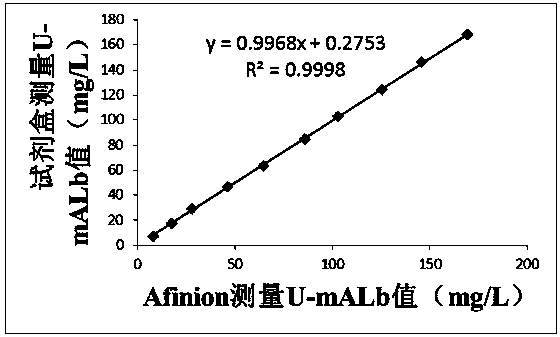
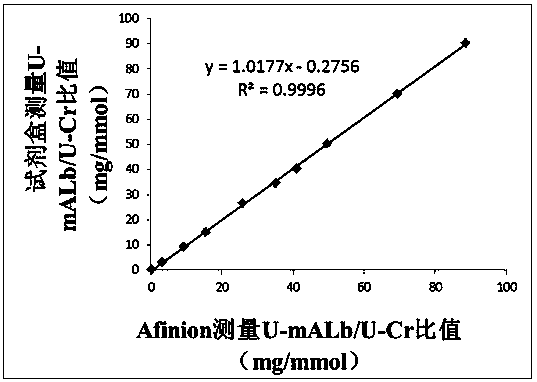
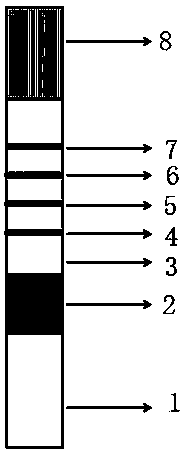
The invention discloses a quantitative immune colloidal gold detection card for urine cystatin C, urine microalbumin and urine creatinine. The detection card comprises a buckle box, a bottom plate, and a sample pad, a combination pad, a chromatographic membrane and a sample absorption pad which are sequentially adhered to the bottom plate. The combination pad is coated with a colloidal gold labeled cystatin C antibody I, a urine microalbumin antibody I, a urine creatinine antibody I and a chicken IgY. A first detection line coated with a cystatin C antibody II, a second detection line coated with a urine microalbumin antibody II, a third detection line coated with a urine creatinine antigen modified by BSA, and a quality control line coated with goat anti-chicken IgY are arranged on the chromatographic membrane. Compared with single detection of U-mALb and U-Cr values, the U-mAlb / U-Cr ratio obtained in the invention can more sensitively reflect the degree of renal function impairment,and the accuracy of the detection result is improved.
Owner:HUNAN UNIV OF TECH
Quantitative detection kit for Sflt-1
InactiveCN111141915AReduce consumptionEnsure safetyDisease diagnosisBiological testingDiseaseBiology
The invention discloses a quantitative detection kit for Sflt-1. The kit comprises a substrate and a cover plate, the substrate and the cover plate are formed by bonding, a sample adding hole and a detection hole are formed in the cover plate, a sample adding area, a reaction area, a detection area and a waste liquid area are sequentially arranged on the substrate, the reaction area is coated witha fluorescent microsphere labeled sFLT-1 antibody I, and the detection area is internally and fixedly coated with sFLT-1 antibodies II with different epitopes. The kit is high in sensitivity, good inrepeatability, extremely small in required sample size, short in detection time and simple and convenient to operate, the operation process can be greatly simplified, the consumption of samples and reagents is reduced, expensive instruments do not need to be arranged, on-site instant detection becomes possible, and the kit has huge economic value and social value; meanwhile, a doctor can be helped to diagnose related diseases of preeclampsia, and early identification and intervention are performed on high-risk groups, so that the safety of maternal and infant in the gestation period is guaranteed.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
A biochip that can expand the scope of immunodetection
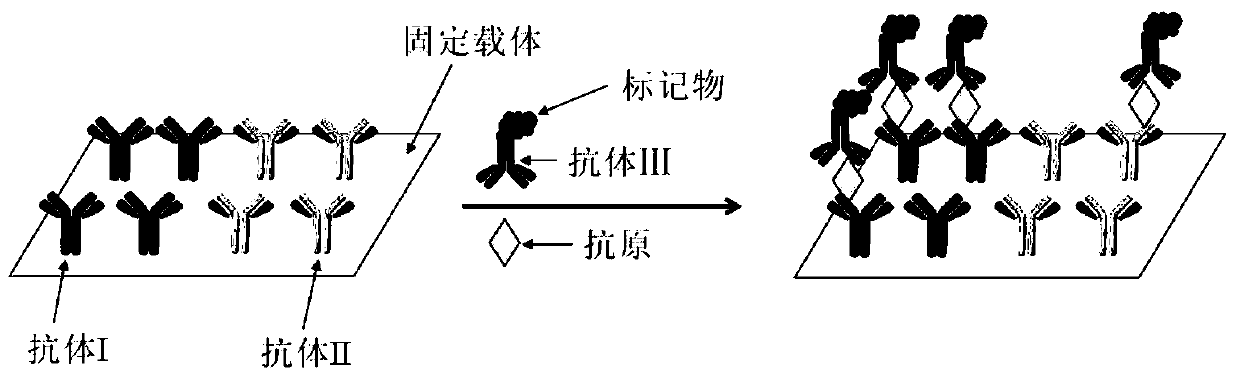
ActiveCN106198955BSimple and fast operationIncreased sensitivityMaterial analysisImmobilized AntibodiesImmuno detection
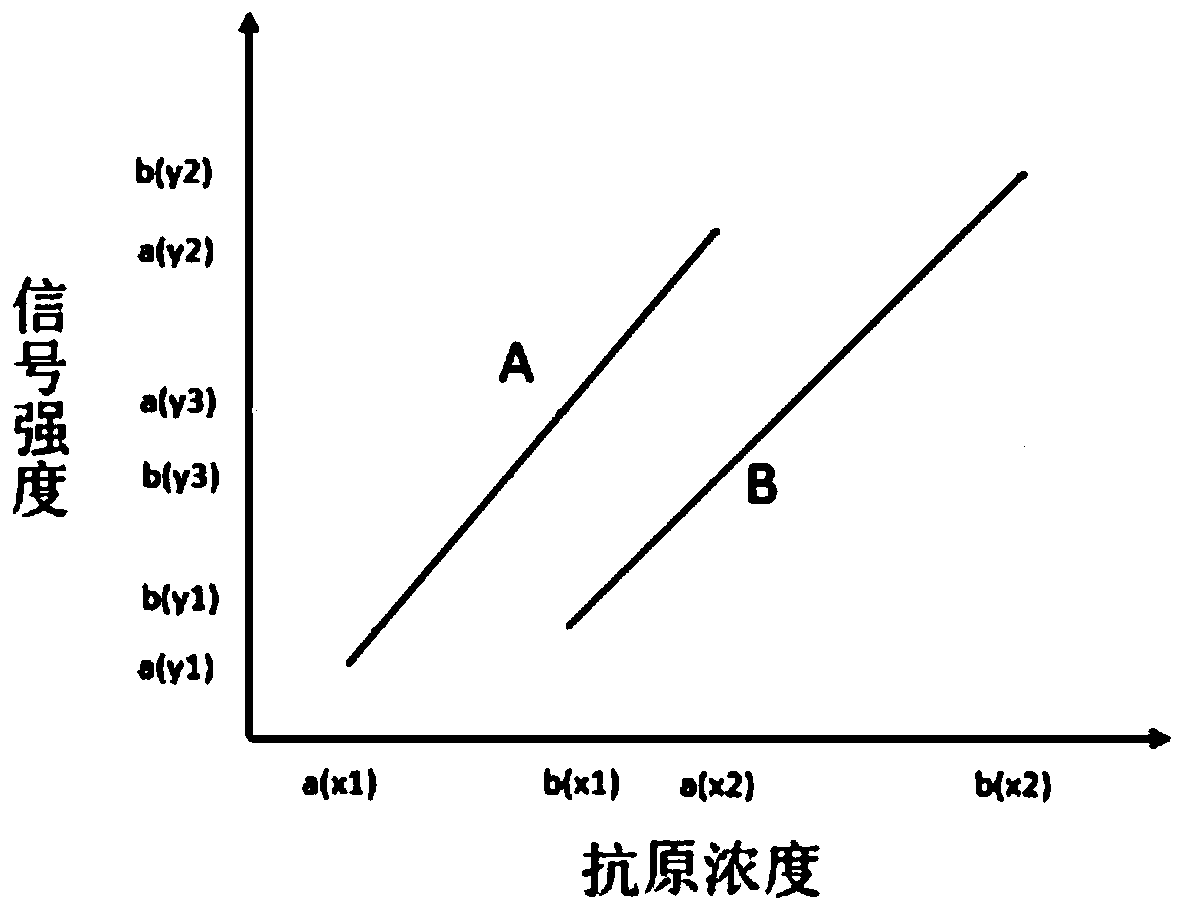
The invention discloses a biochip being able to widen the immunodetection range. The biochip comprises two or above distinguishable detection areas, the detection areas can immobilize an antibody I and an antibody II, and the antibody I and the antibody II are combined with a same antigen. The chip has the advantages of simplicity in operation, high sensitivity, wide detection range, effective widening of the immunodetection range of a sample, and improvement of the detection precision.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI +1
Luminol chemiluminescence immunological analysis detecting method for cardiac muscle calcium protein
The present invention relates to clinical blood detecting and analyzing technology, and is the cTnI Luminol chemiluminescence immunological analysis detecting method with the system including self made cardiac muscle calcium protein resisting antibody I(cTnI) to label horseradish peroxidase, cinnamic acid as luminescence intensifier, tetreaphenyl sodium borate as synergistic intensifier and horseradish peroxidase as catalyst for Luminol-H2O2 luminescence. Compared with available ELISA process, the said CLIA process of the present invention has no obvious difference, correlation coefficient of 0.9852 and diagnosis coincidence rate of 96.15 %.
Owner:CHINA PHARM UNIV
Kit for rapidly and quantitatively assaying PD-L1 (programmed death ligand-1) by chemiluminescent magnetic bead enzyme-linked immunosorbent assay
InactiveCN108152484AStrong specificityImprove stabilityChemiluminescene/bioluminescenceBiological testingMagnetic beadPD-L1
The invention belongs to the technical field of assay, and particularly relates to a kit for rapidly and quantitatively assaying PD-L1 (programmed death ligand-1) by chemiluminescent magnetic bead enzyme-linked immunosorbent assay. The kit for rapidly and quantitatively assaying PD-L1 by chemiluminescent magnetic bead enzyme-linked immunosorbent assay disclosed by the invention consists of standards, mouse anti-human PD-L1 antibody I-coated immunomagnetic beads, an enzyme-labeled mouse anti-human PD-L1 antibody II, chemiluminescent substrates, and auxiliary reagents. The kit for rapidly and quantitatively assaying PD-L1 by chemiluminescent magnetic bead enzyme-linked immunosorbent assay disclosed by the invention has the advantages of high specificity, good stability, high sensitivity andlower cost, and is suitable for large-scale clinic popularization.
Owner:珠海霍普金斯医药研究院股份有限公司
A kind of immunohistochemical operation method of tissue section
Owner:SICHUAN KINGMED DIAGNOSTICS CENT
NT-proBNP quantitative detection kit and preparation method thereof
PendingCN112858710AQuick checkHigh detection sensitivityLaboratory glasswaresBiological testingBlood filmPhysical chemistry
The invention relates to an NT-proBNP quantitative detection kit and a preparation method thereof, the NT-proBNP quantitative detection kit comprises a chip and a reagent, and the chip comprises a chip substrate and a chip cover plate; a sample adding area, a reaction area, a detection area and a waste liquid pool are sequentially arranged on the chip substrate; the sample adding area is communicated with the reaction area through a filtering channel, and a blood filtering film is arranged in the filtering channel; the reaction area is communicated with the detection area through a reaction channel, a first flow limiting column is arranged in the reaction channel, the first flow limiting column is of a bent structure, and the bending direction extends in the width direction of the reaction channel; the detection area is communicated with the waste liquid pool through a waste liquid channel, a second flow limiting column is arranged at the communication position of the waste liquid channel and the waste liquid pool and is of a wave-shaped structure, and a protrusion is arranged at the end, facing the waste liquid pool, of the second flow limiting column; the reaction area is coated with an NT-proBNP antibody I marked by fluorescent microspheres, and an NT-proBNP antibody II is fixed in the detection area; a sample adding hole, a detection window and an air hole are formed in the chip cover plate. The kit has the advantages of rapidness, simplicity and convenience in detection and capability of greatly improving the detection sensitivity and accuracy.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
A kit for early prediction of osteoporosis
ActiveCN105759053BIt has the function of risk predictionEasy to operateDisease diagnosisBiological testingBone densitySerum samples
The invention provides a kit for early prediction of osteoporosis. The kit comprises a detection plate, a standard substance, interferon-gamma antibodies I and interferon-gamma antibodies II capable of emitting fluorescent light, wherein the detection plate comprises a plate body and at least two sample adding holes in the plate body; the bottoms of the sample adding holes are coated with the interferon-gamma antibodies II; the standard substance is an interferon-gamma water solution, and the concentration of the water solution is higher than or equal to 1 ng / mL. A serum sample of a to-be-tested person and the standard substance are added to the sample adding holes in the detection plate respectively, and whether the to-be-tested person has the risk of osteoporosis is judged according to the difference of fluorescence intensity. The kit is low in cost, high in accuracy and good in reliability, has a risk prediction function by comparison with traditional bone density examination and can provide a diagnosis basis for prediction of osteoporosis in clinic.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Plasmodium falciparum latex process detection kit
InactiveCN106290839AHigh sensitivityStrong specificityBiological material analysisBiological testingReagent stripPolyester
The invention relates to the field of latex process immunochromatography, and particularly discloses a plasmodium falciparum latex process detection kit. The kit comprises a reagent strip, wherein the reagent strip comprises a substrate, filtering sample paper, a sensitized latex polyester membrane, an immune nitrocellulose membrane and water absorption paper, wherein the filtering sample paper, the sensitized latex polyester membrane, the immune nitrocellulose membrane and the water absorption paper are sequentially in end-to-end connection and are fixed on the substrate; color latex particle-marked plasmodium falciparum-resistant histidine enriched protein II monoclonal antibodies I cover the sensitized latex polyester membrane; detection lines coated by plasmodium falciparum-resistant histidine enriched protein II monoclonal antibodies II and quality control lines coated with goat anti-rabbit IgG are arranged on the immune nitrocellulose membrane. The plasmodium falciparum latex process detection kit has the advantages that the response is sensitive; the detection is convenient; the detection result is stable; the cost is reduced.
Owner:SHANGHAI CHEMTRON BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com