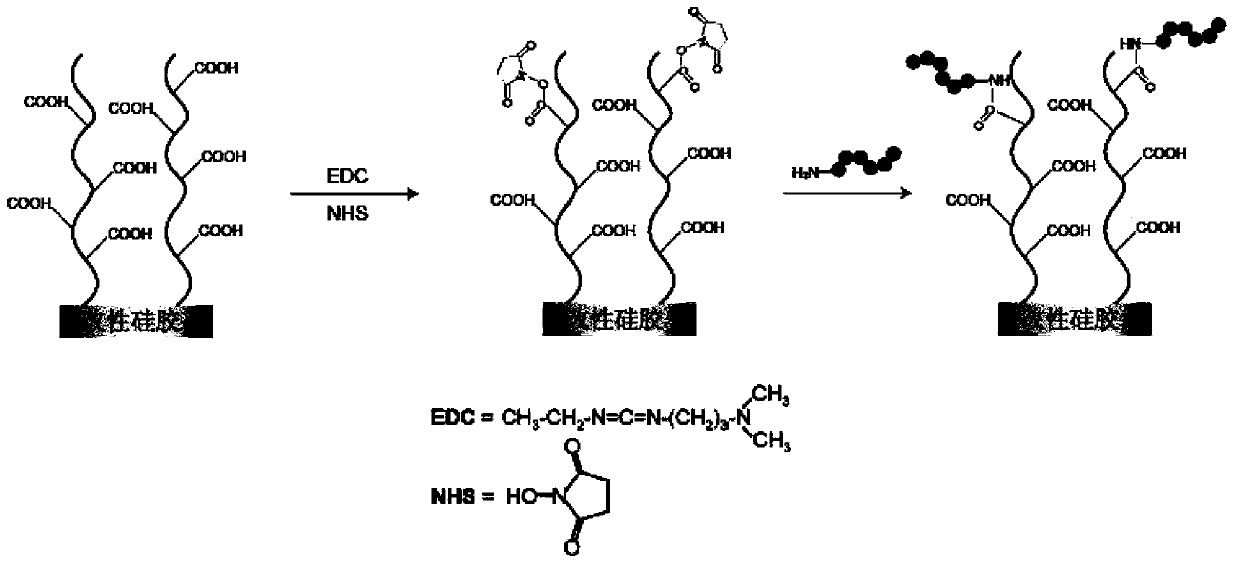
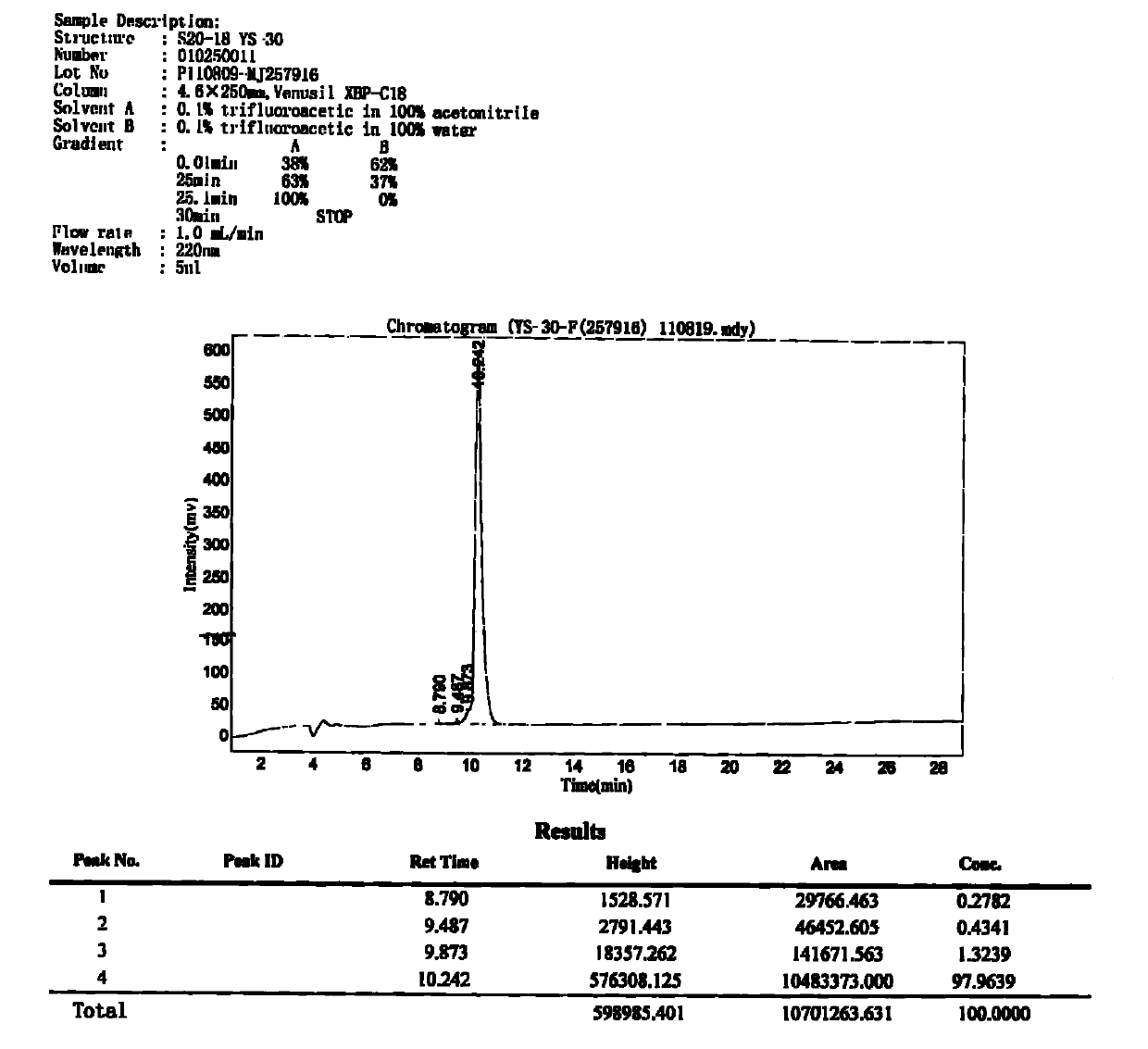
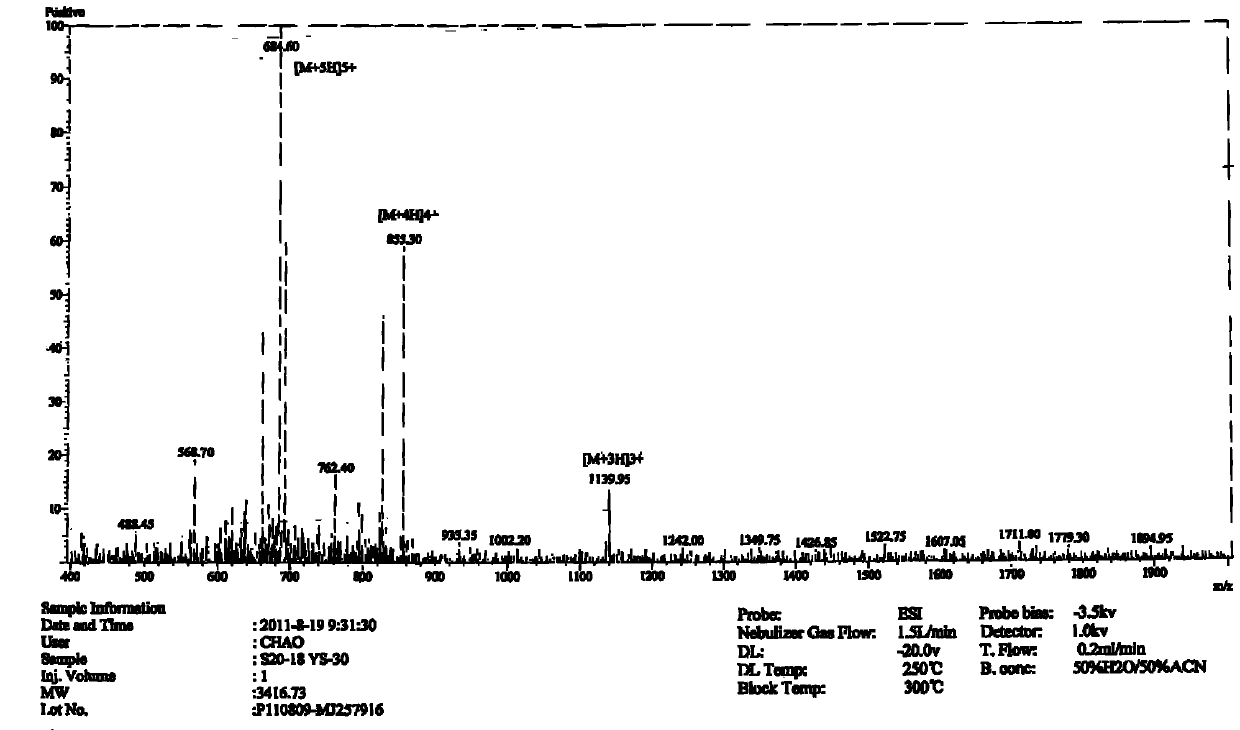
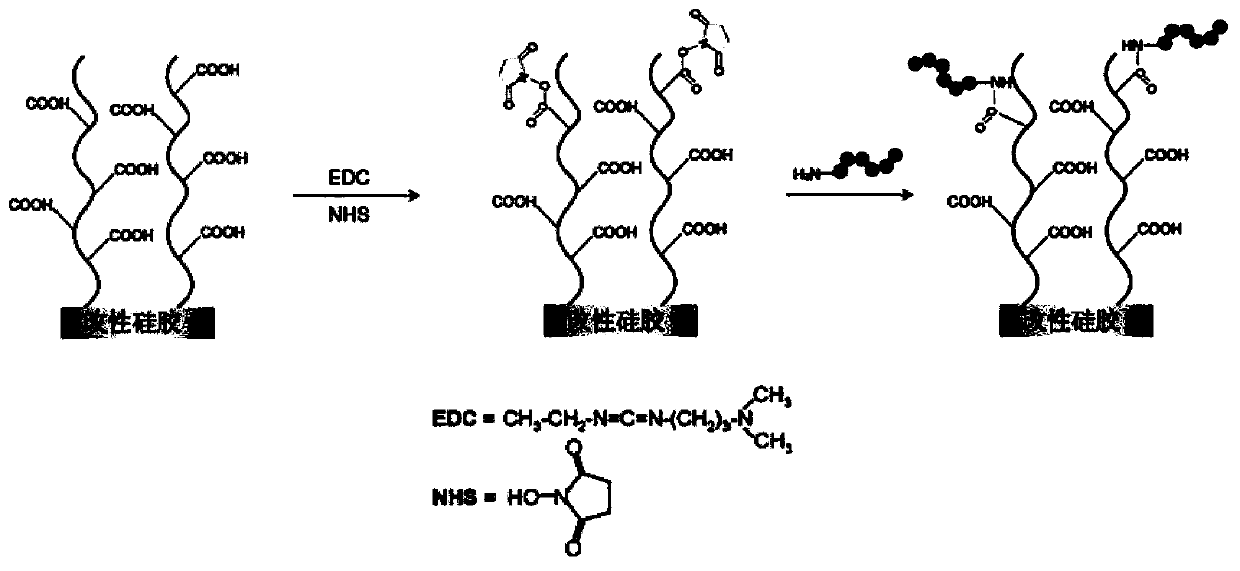
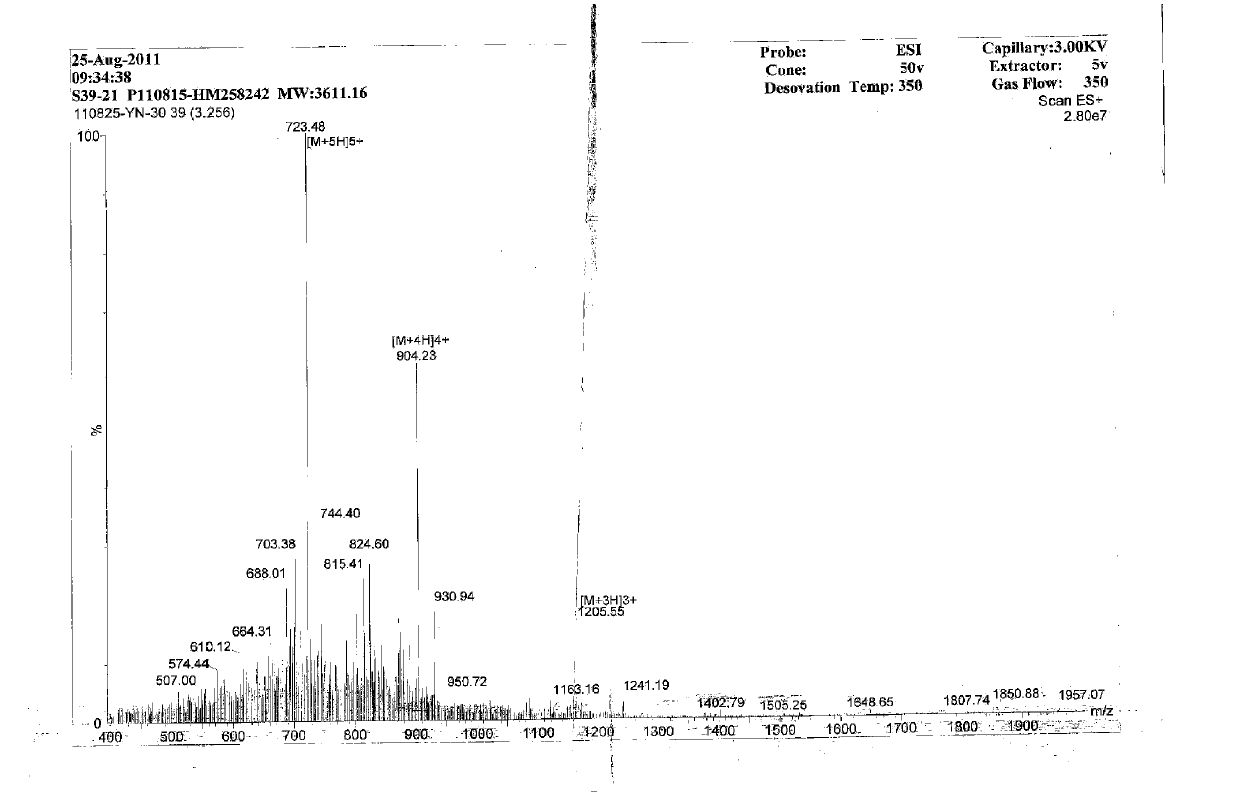
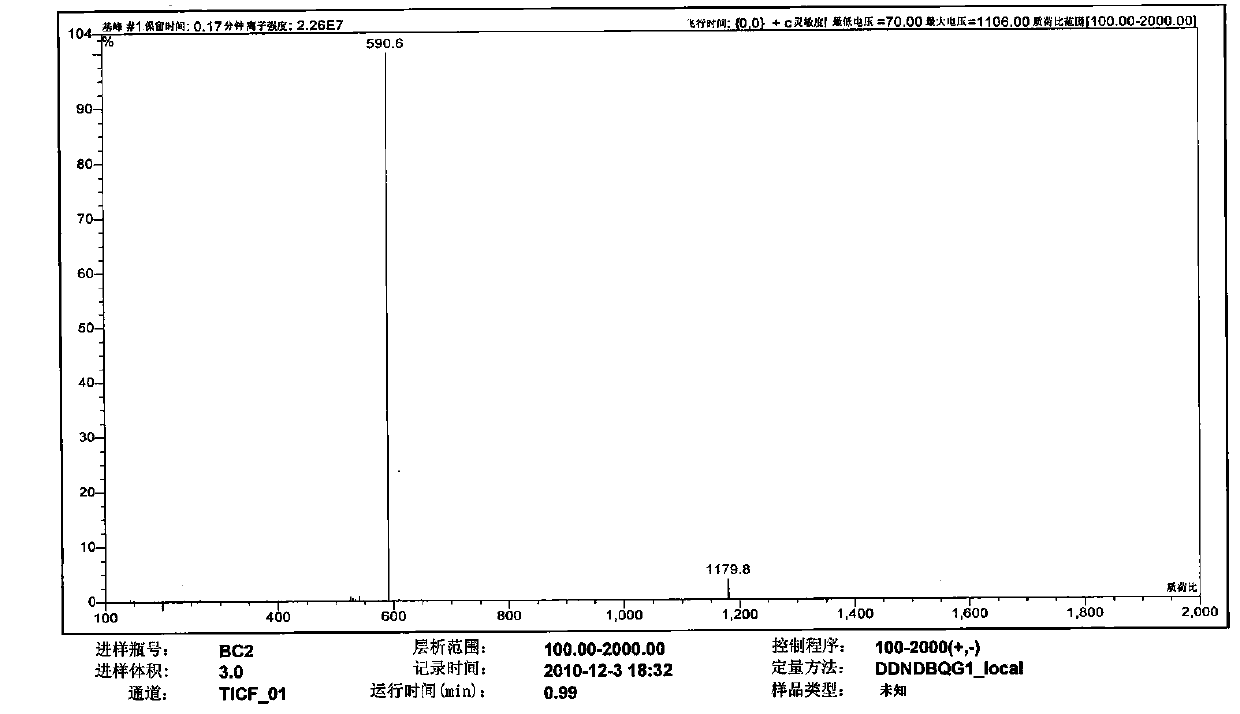
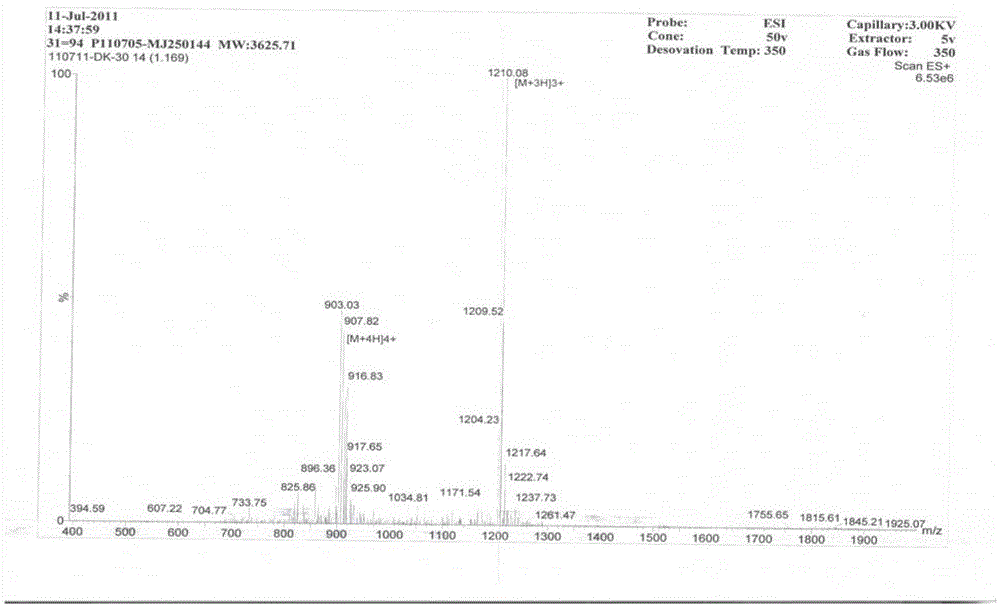
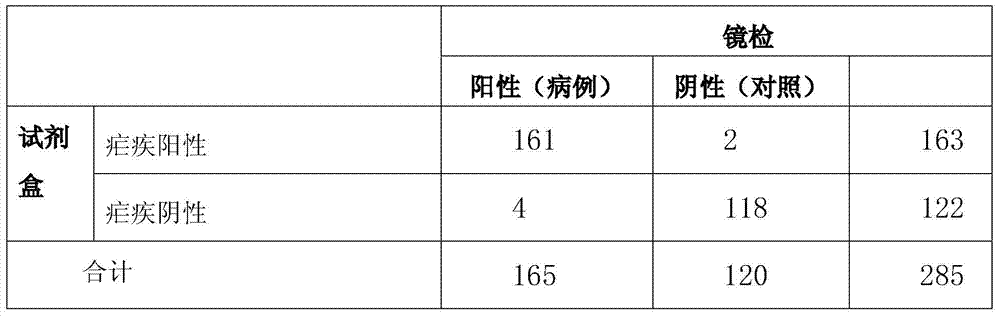
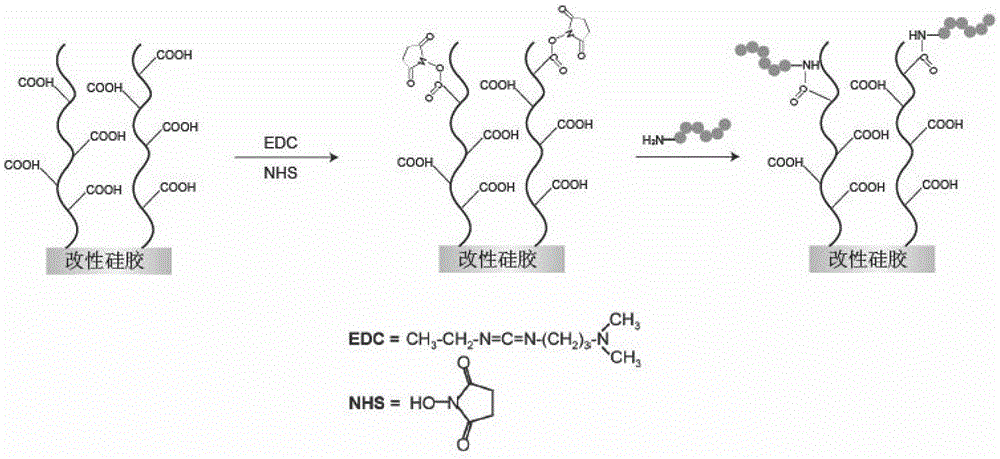
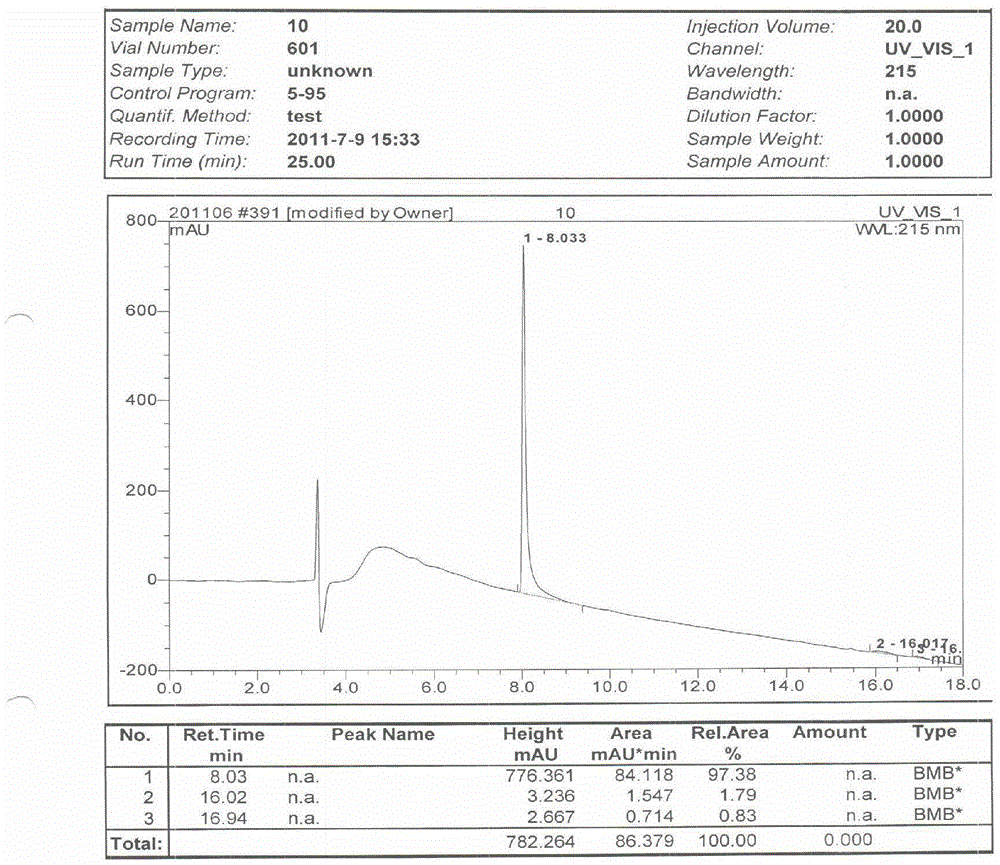
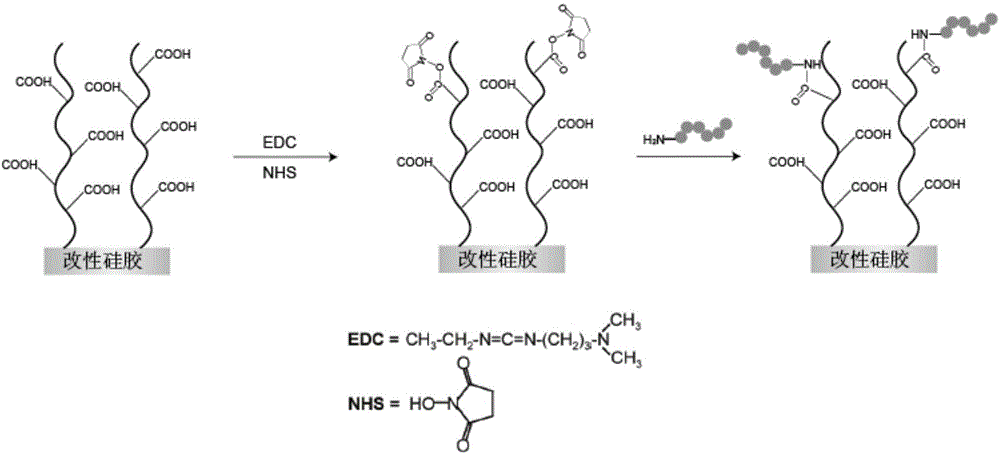
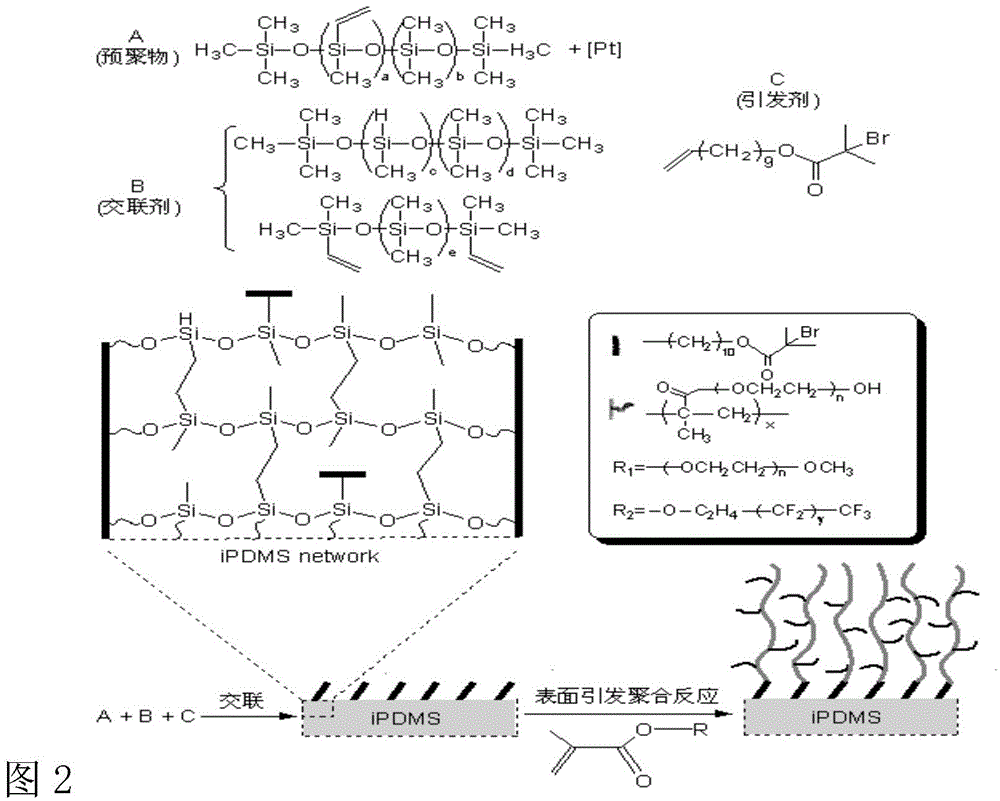
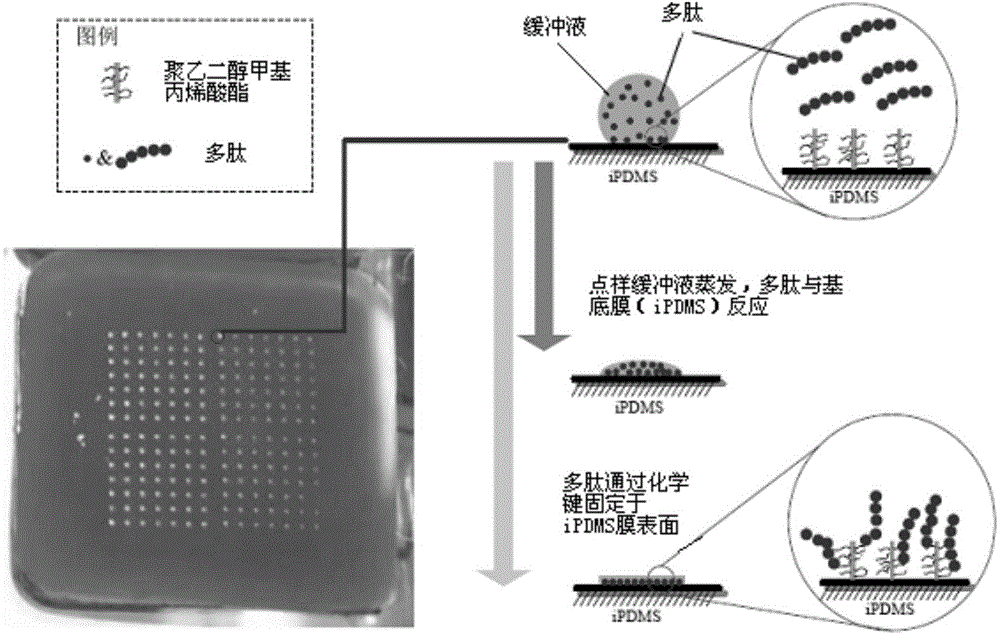
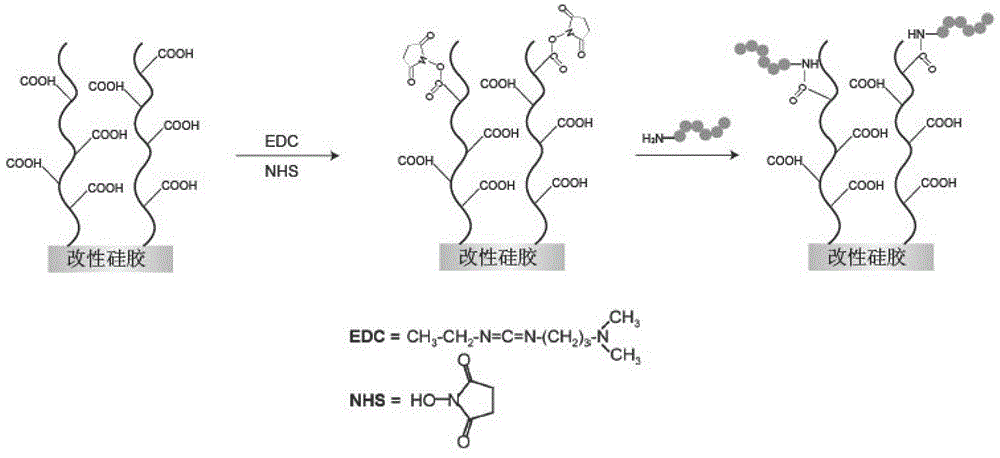
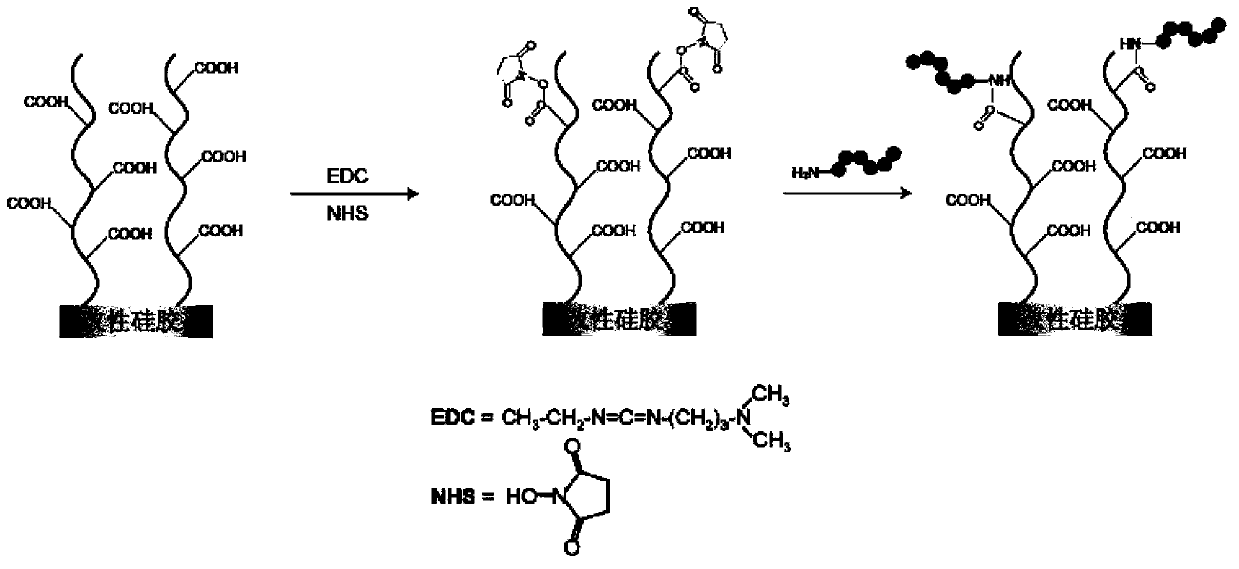
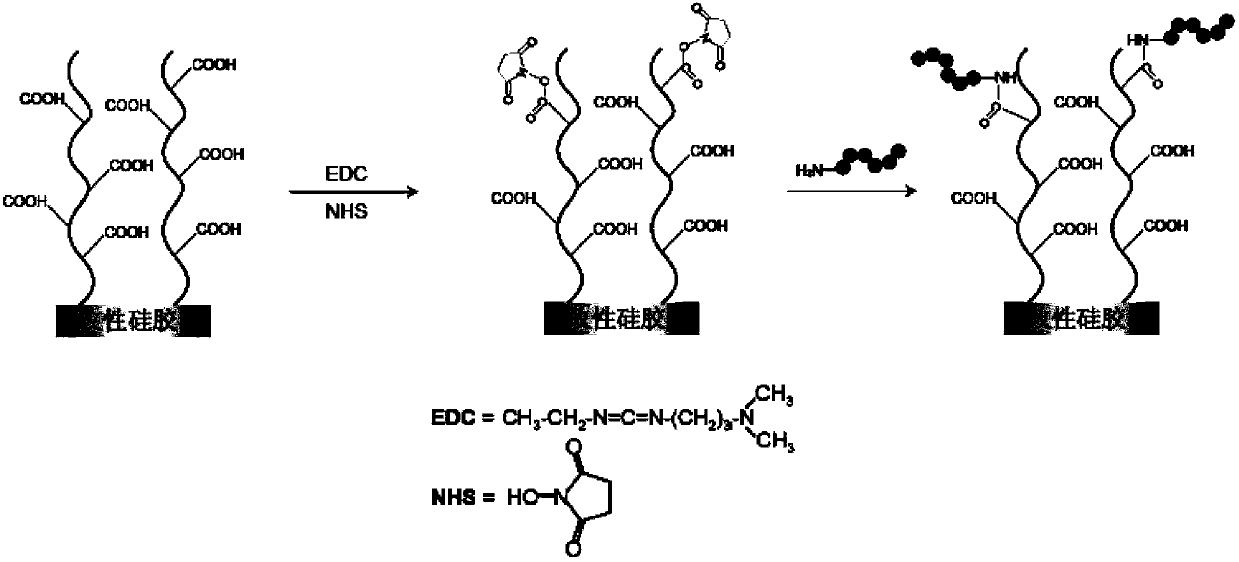
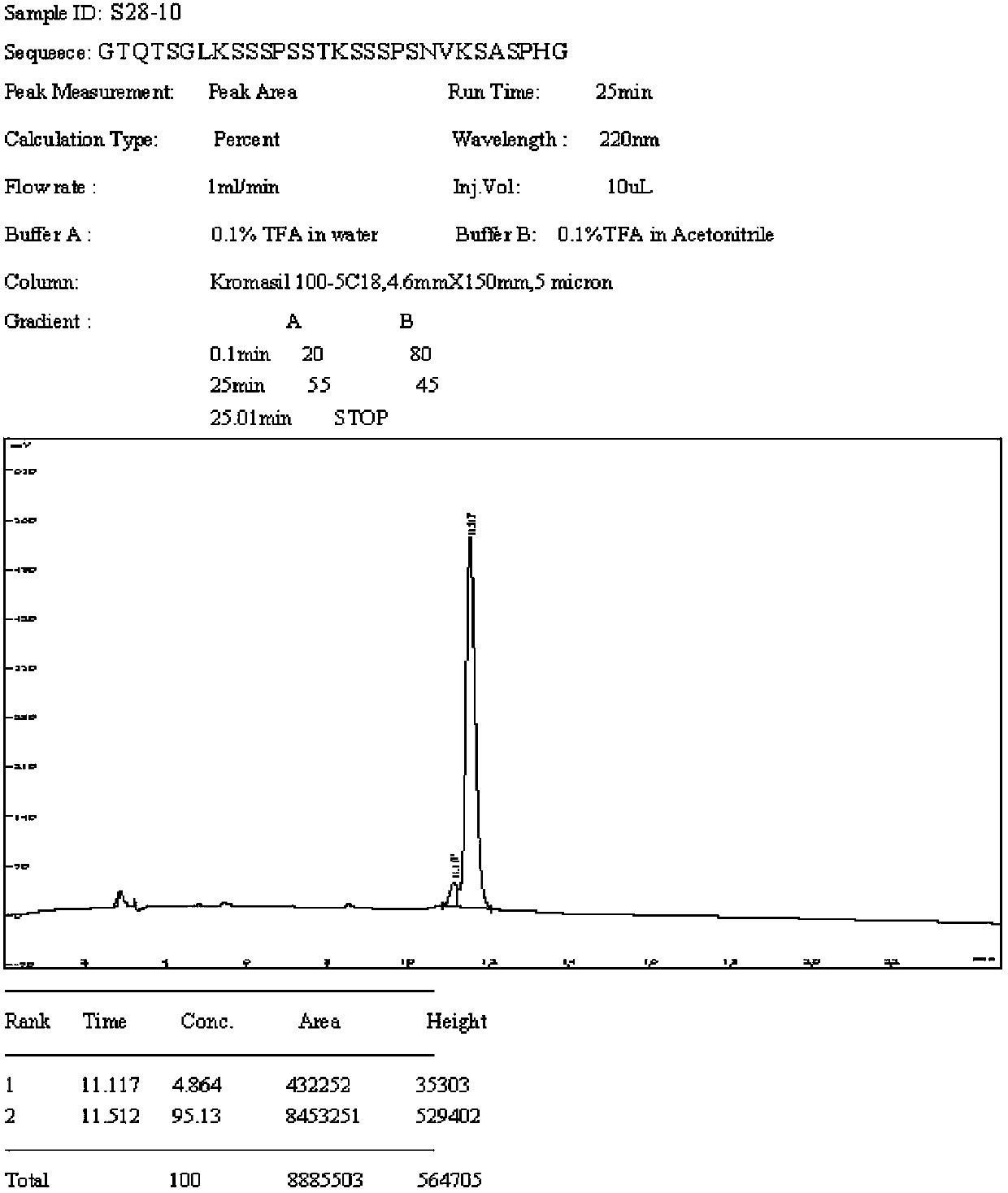
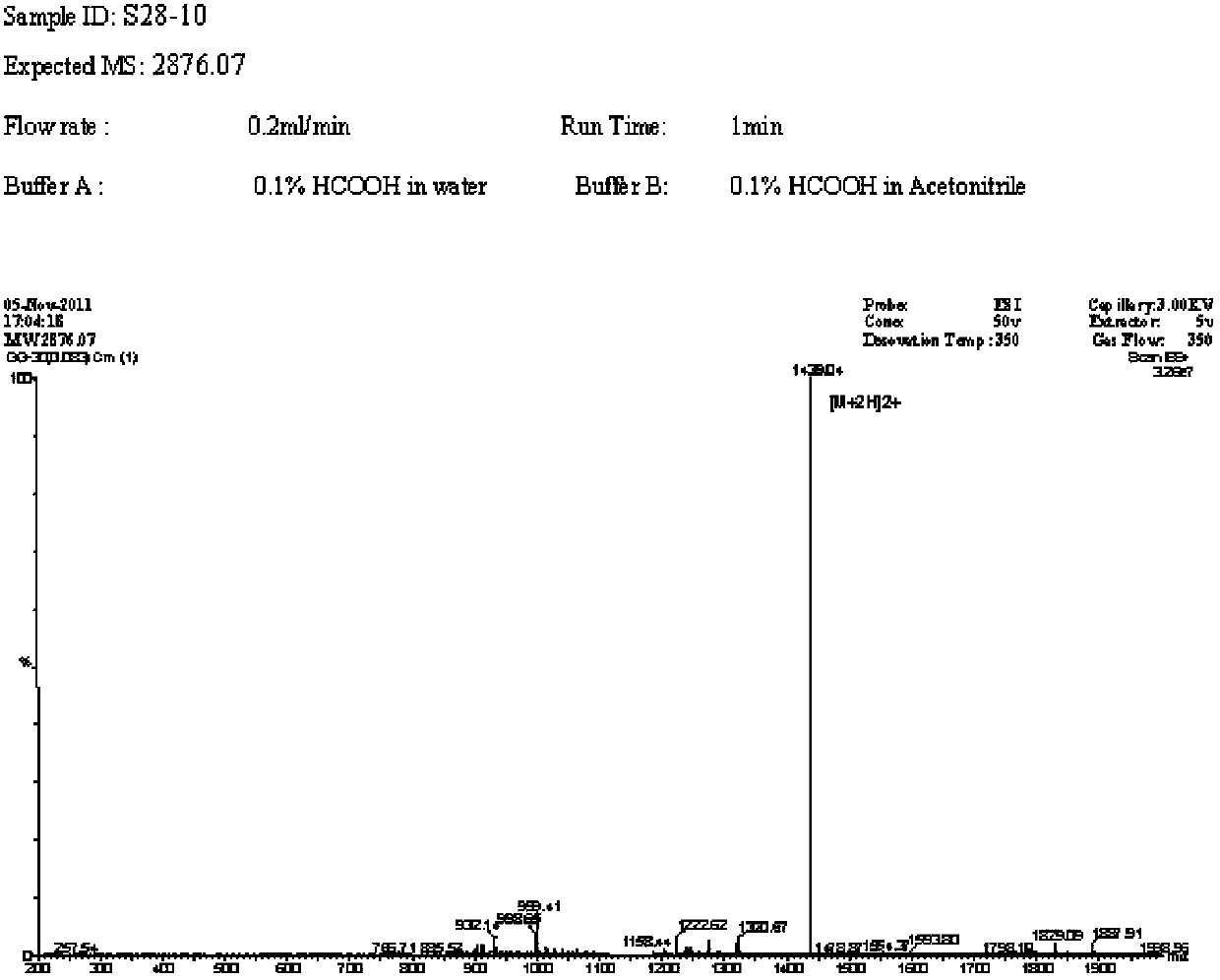
Patents
Literature
67 results about "Diagnosis of malaria" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The mainstay of malaria diagnosis has been the microscopic examination of blood, utilizing blood films. Although blood is the sample most frequently used to make a diagnosis, both saliva and urine have been investigated as alternative, less invasive specimens. More recently, modern techniques utilizing antigen tests or polymerase chain reaction have been discovered, though these are not widely implemented in malaria endemic regions. Areas that cannot afford laboratory diagnostic tests often use only a history of subjective fever as the indication to treat for malaria.
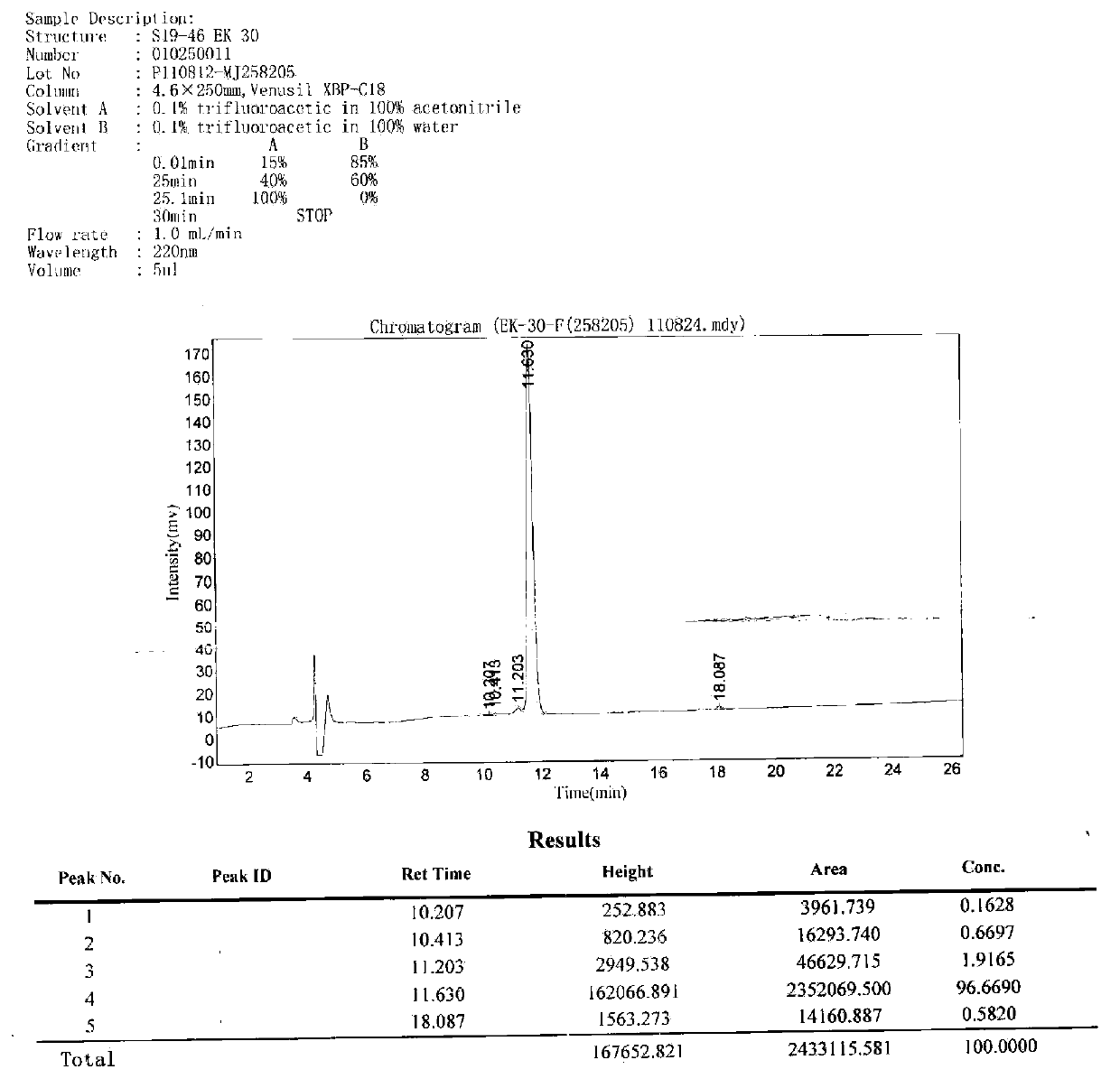
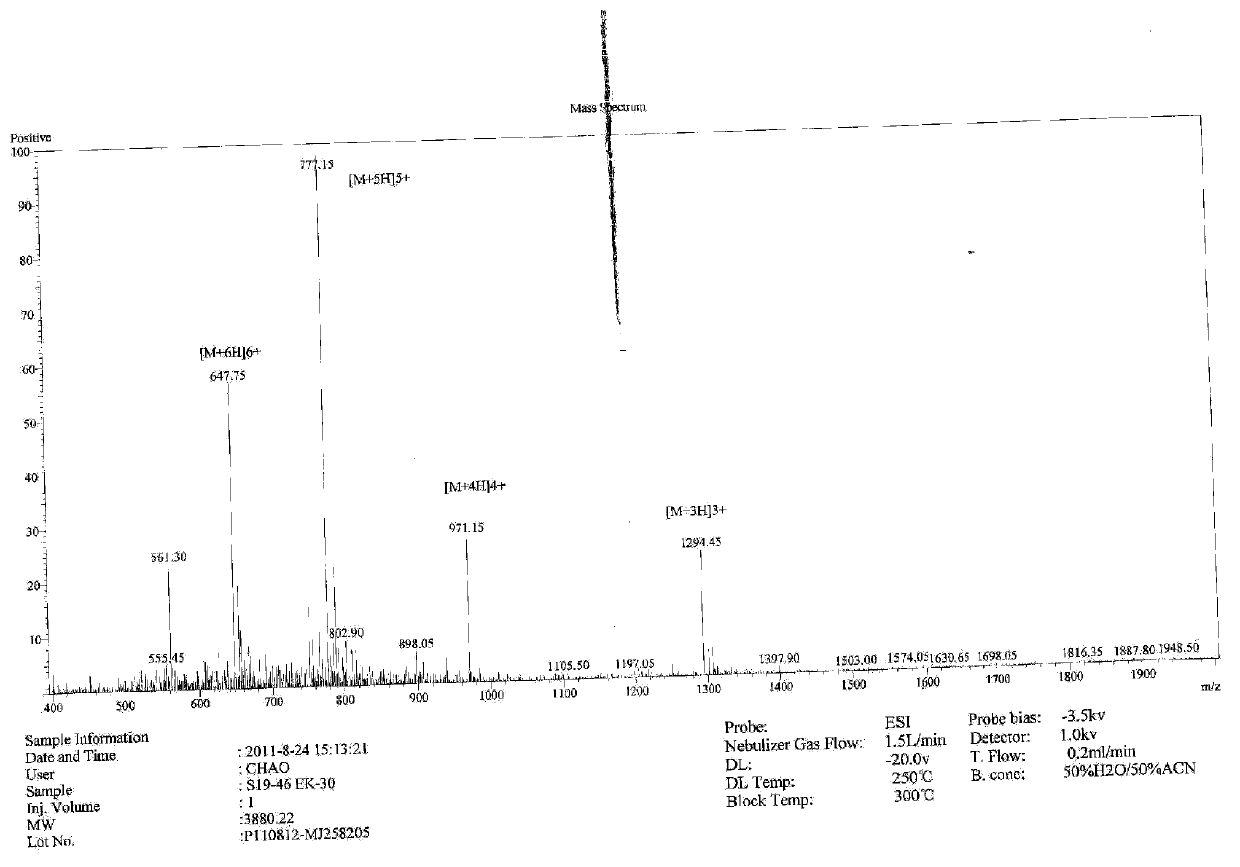
Immunity-chromatography kit for rapid diagnosis of malaria and its pathogen species and preparation method thereof
ActiveCN102103141AThe detection method is simpleObservation is quick and intuitiveTissue cultureImmunoglobulins against enzymesOperating instructionCellulose
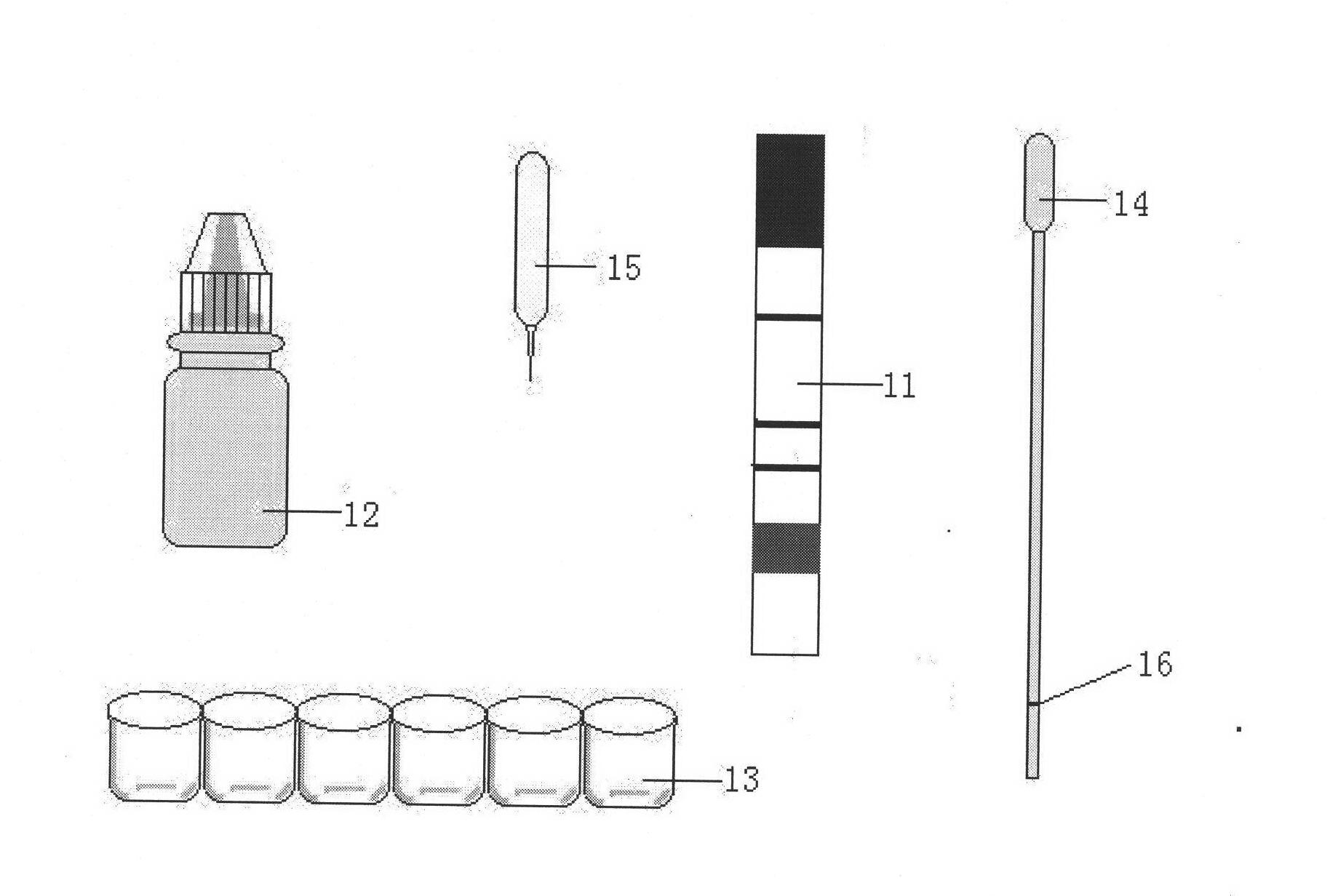
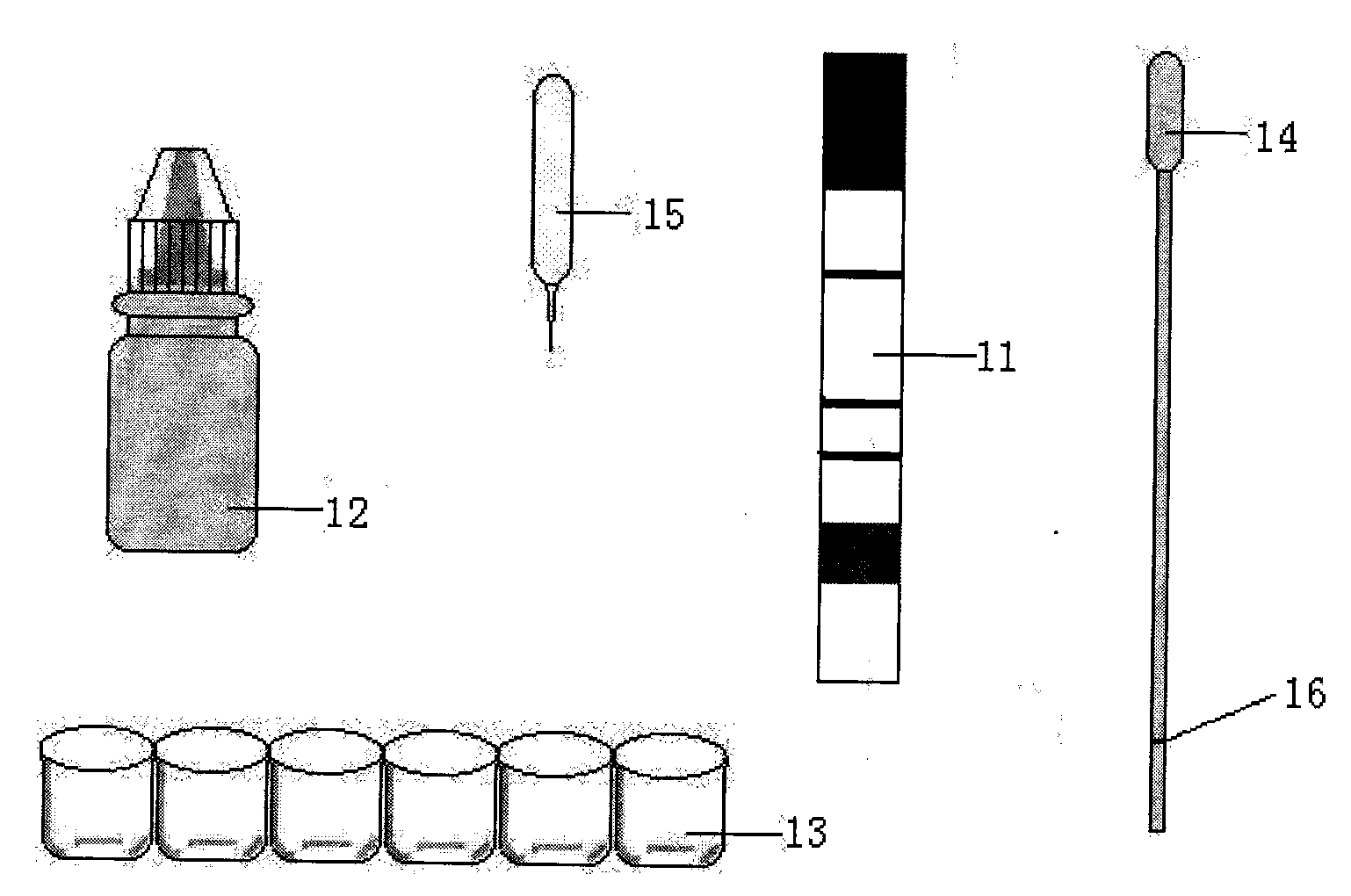
The invention discloses an immunity-chromatography kit for the rapid diagnosis of malaria and its pathogen species and a preparation method thereof. The kit comprises a colloidal gold immunity-chromatography test strip, a matching cell-free lysate, a sample cup, a blood taking needle, and an operating instruction. The colloidal gold immunity-chromatography test strip comprises a sample pad, a colloidal-gold pad, a cellulose membrane, and a water-absorbing pad, wherein the colloidal-gold pad contains colloidal gold labelled antibodies; a detection line and a quality control line are disposed on the cellulose membrane; and the colloidal gold labelled antibody and the detection line are composed of monoclonal antibodies which can specifically bind the lactate dehydrogenase of plasmodia. The immunity-chromatography kit for the rapid diagnosis of malaria and its pathogen of the invention can distinguish falciparum malaria from vivax malaria, has the advantages of simplicity, sensitivity, specificity, and rapidity, and is suitable for clinical and field applications.
Owner:SHANGHAI NEW JIEER CLEANING PRODS
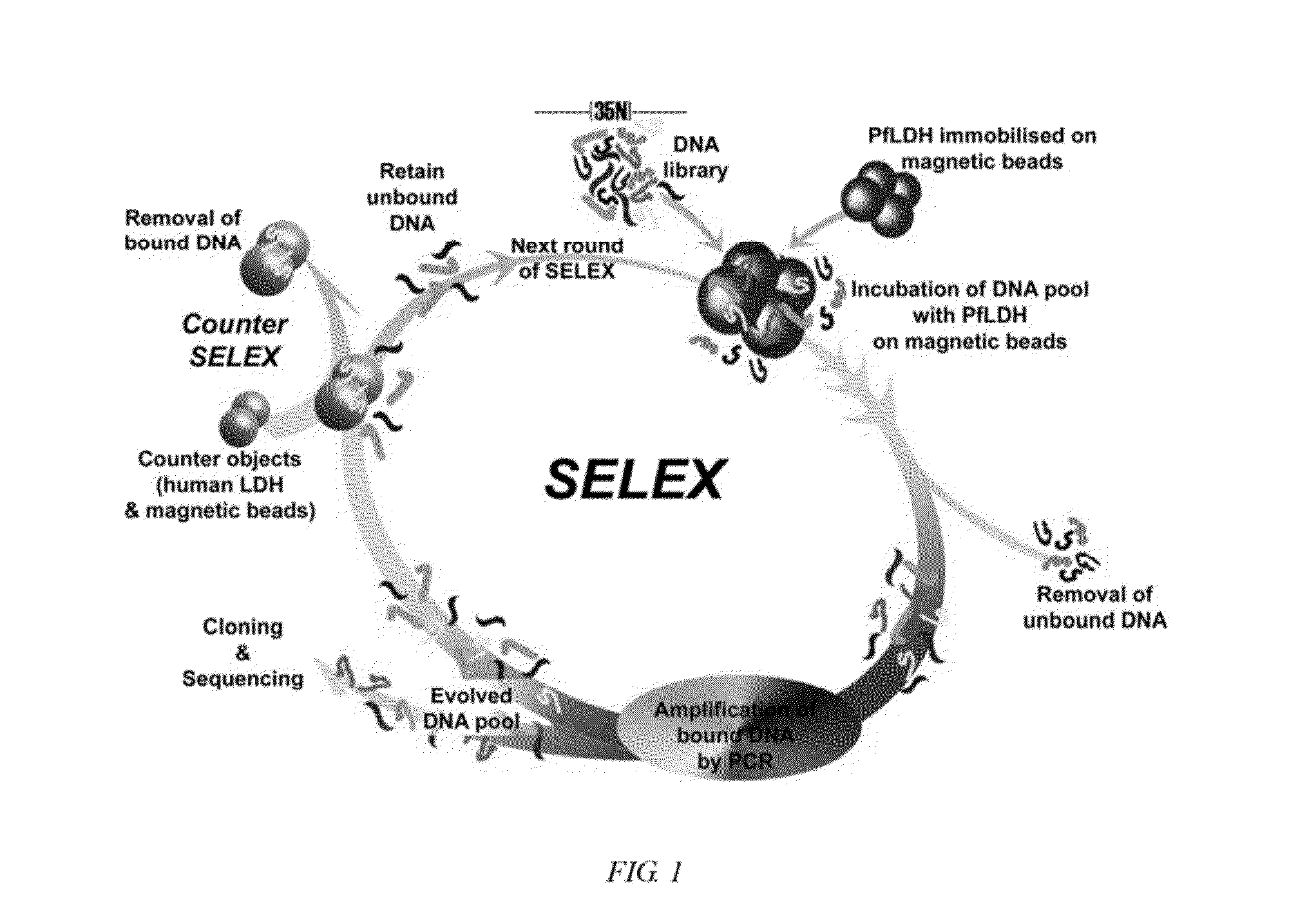
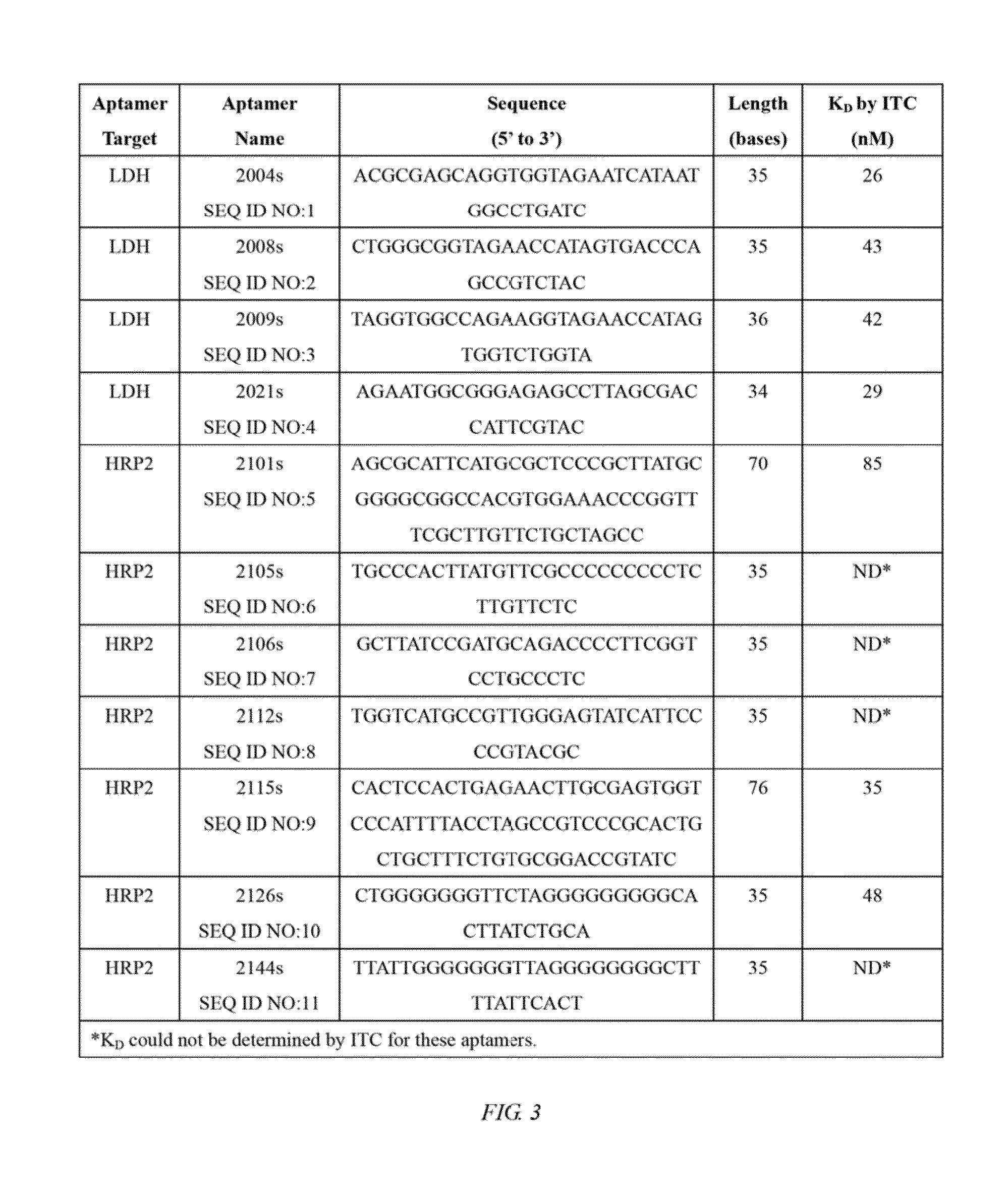
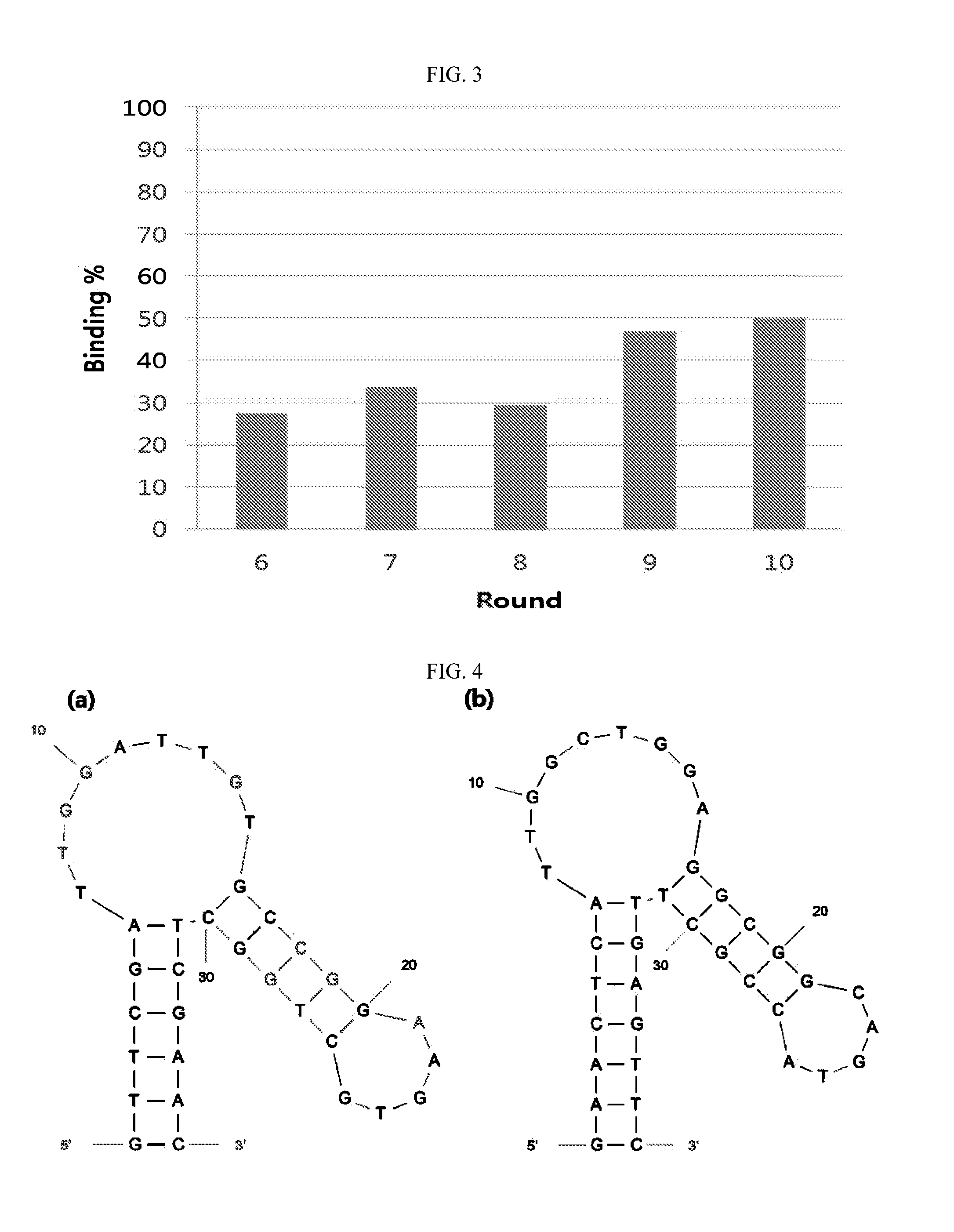
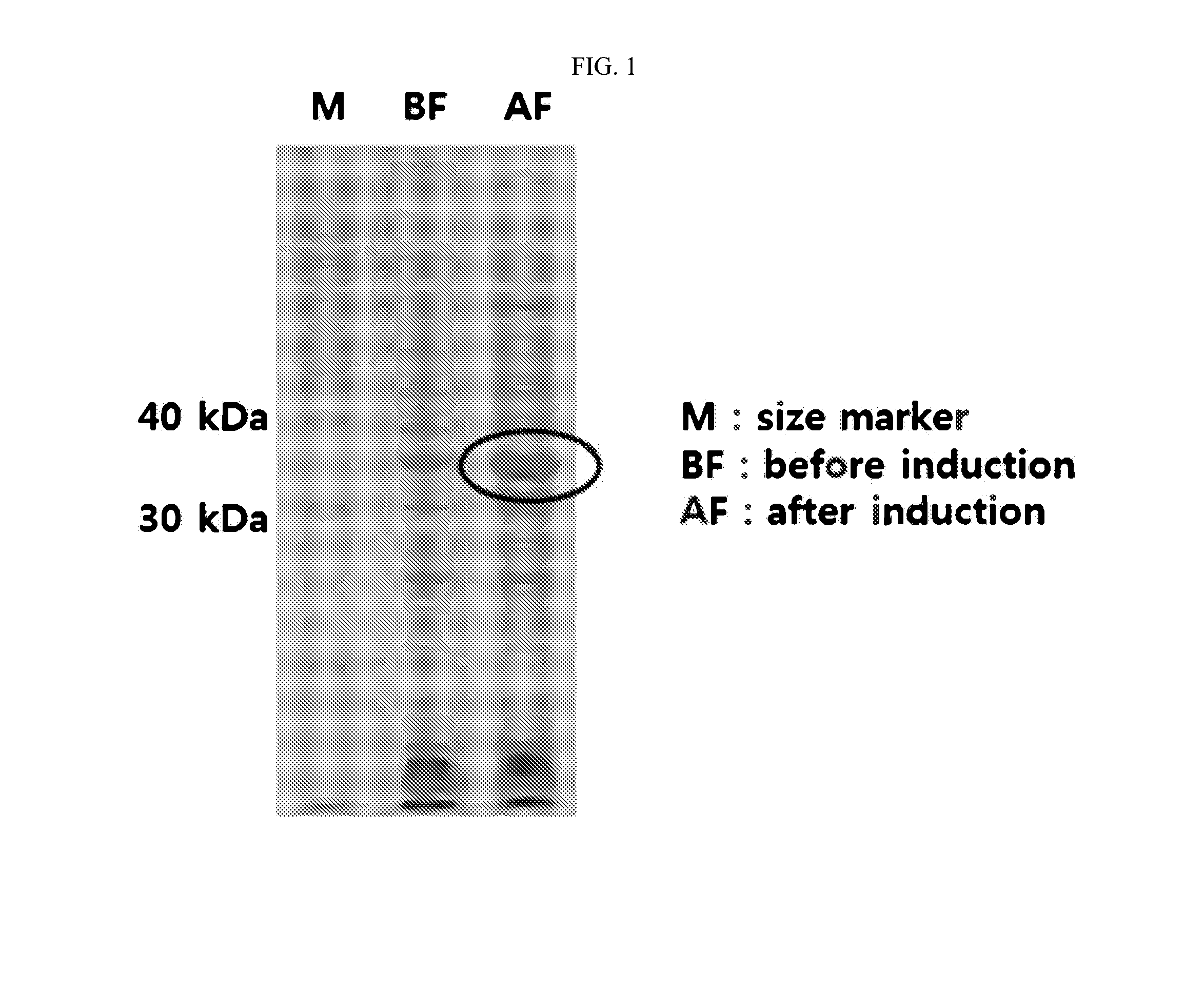
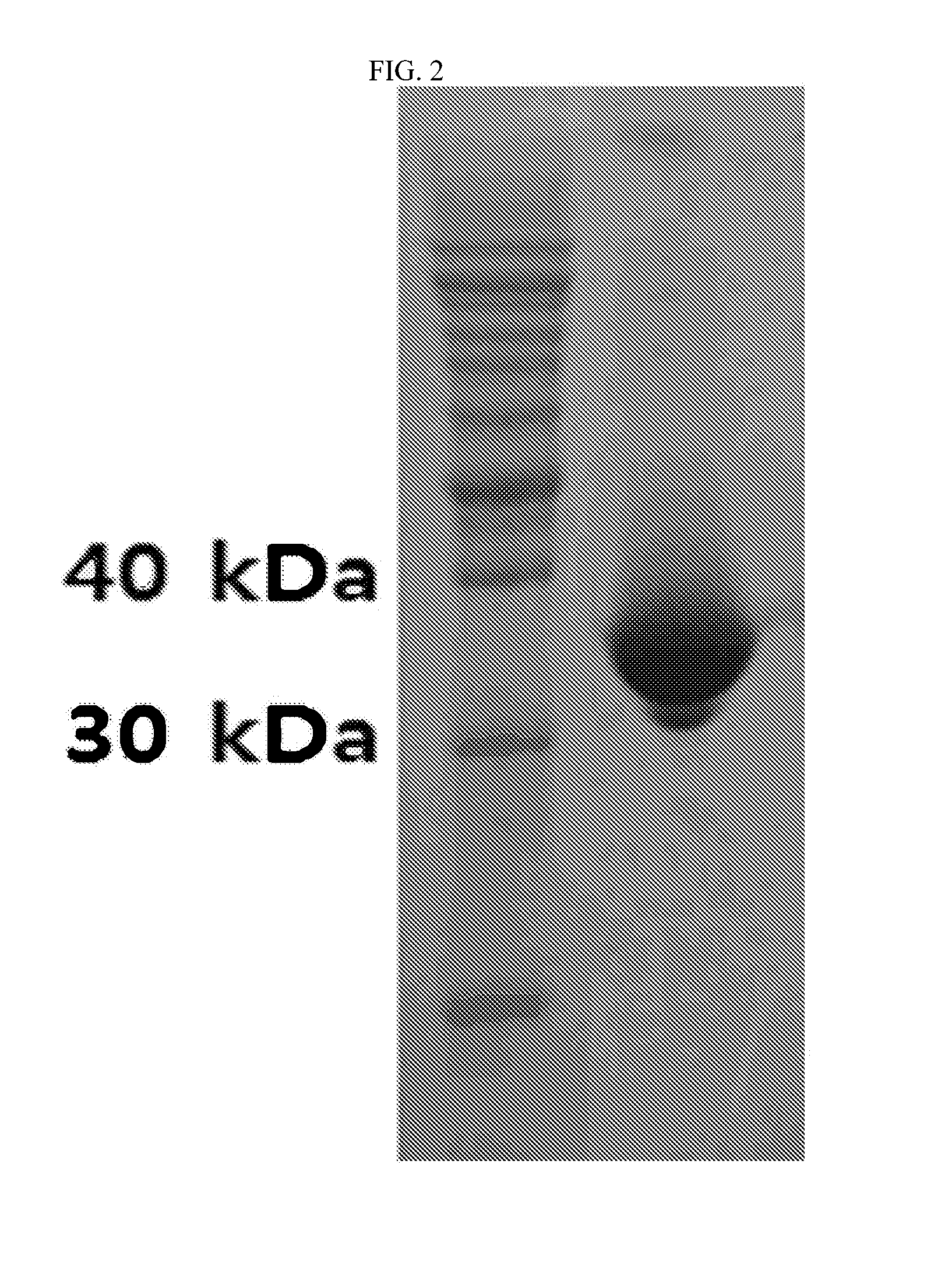
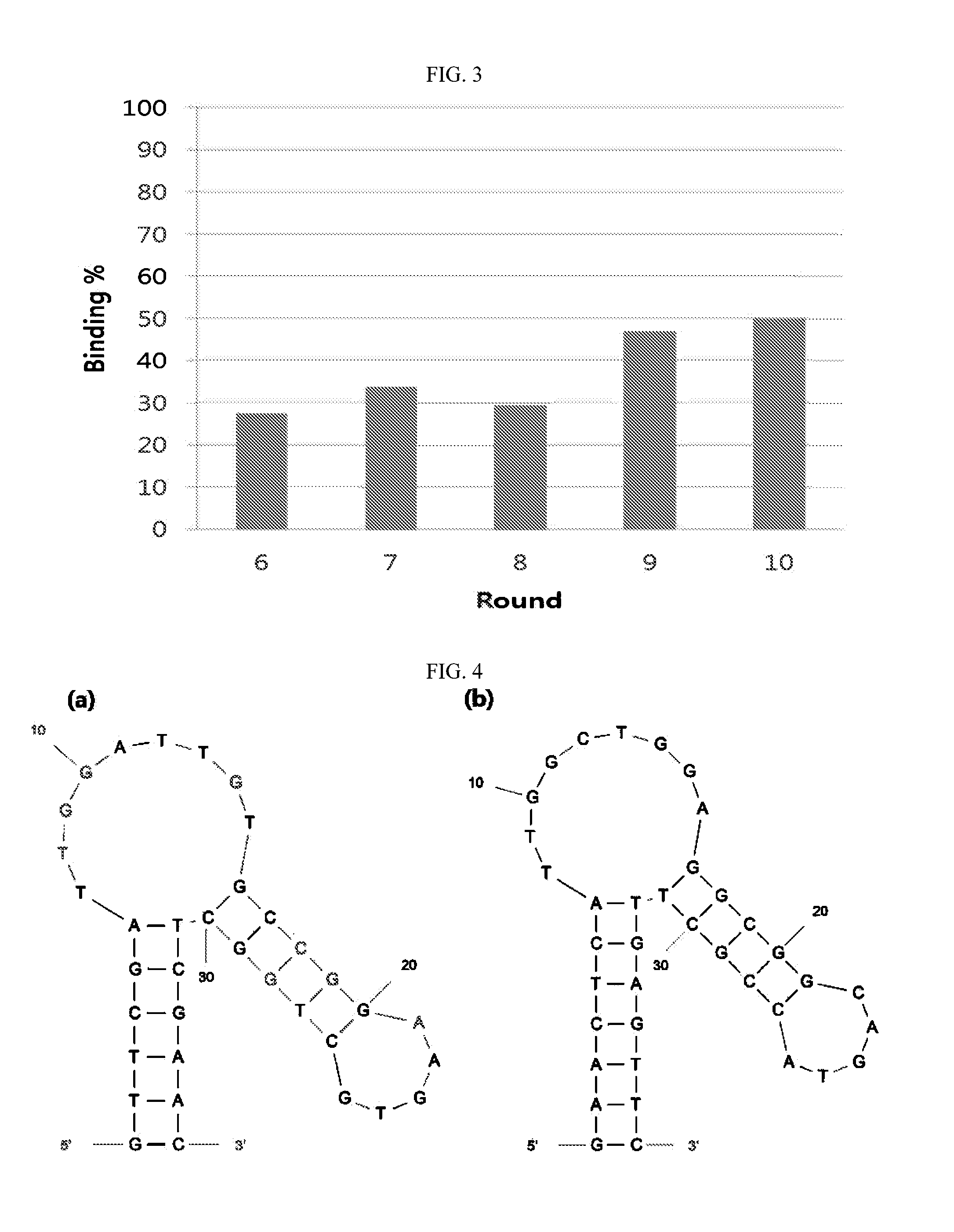
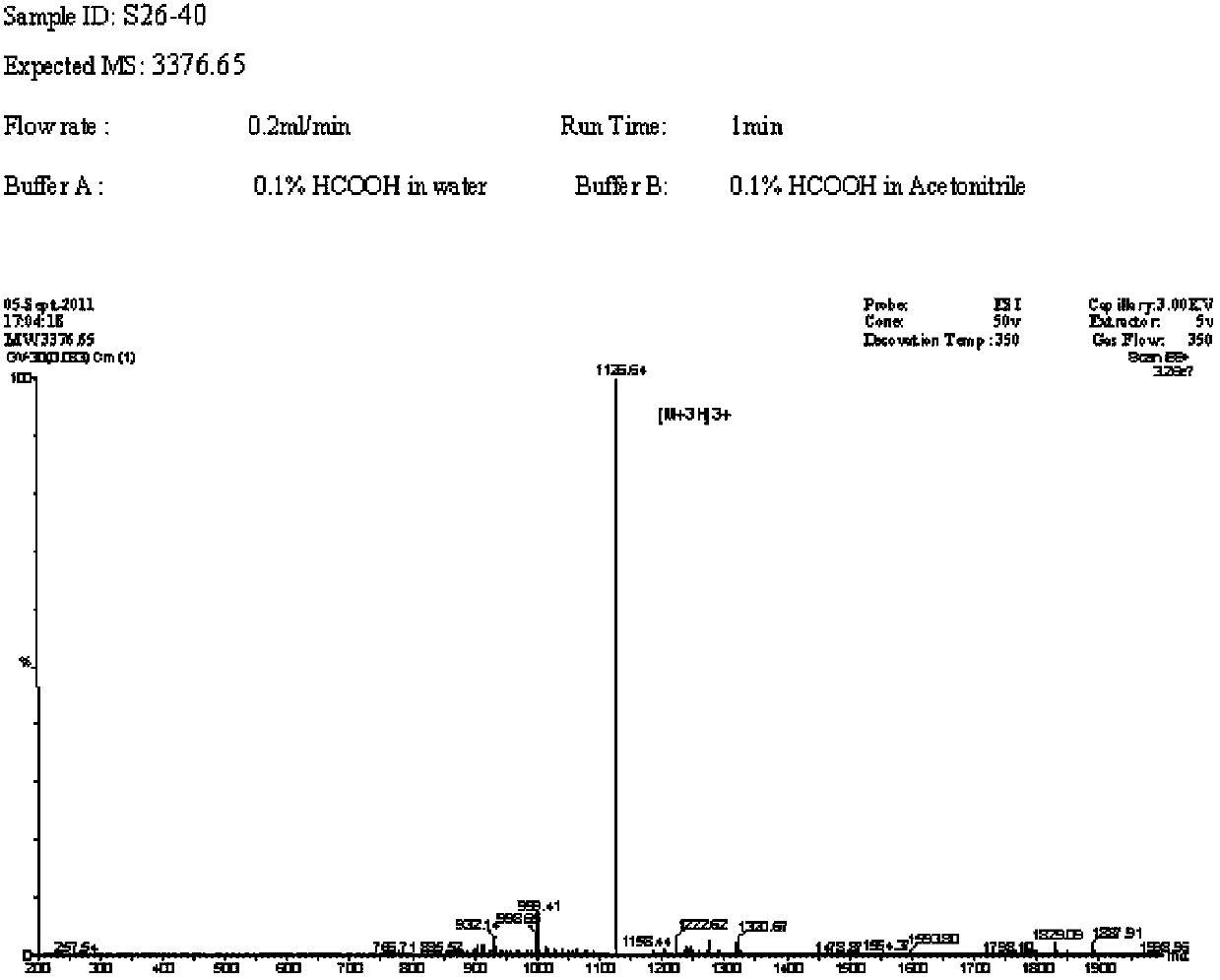
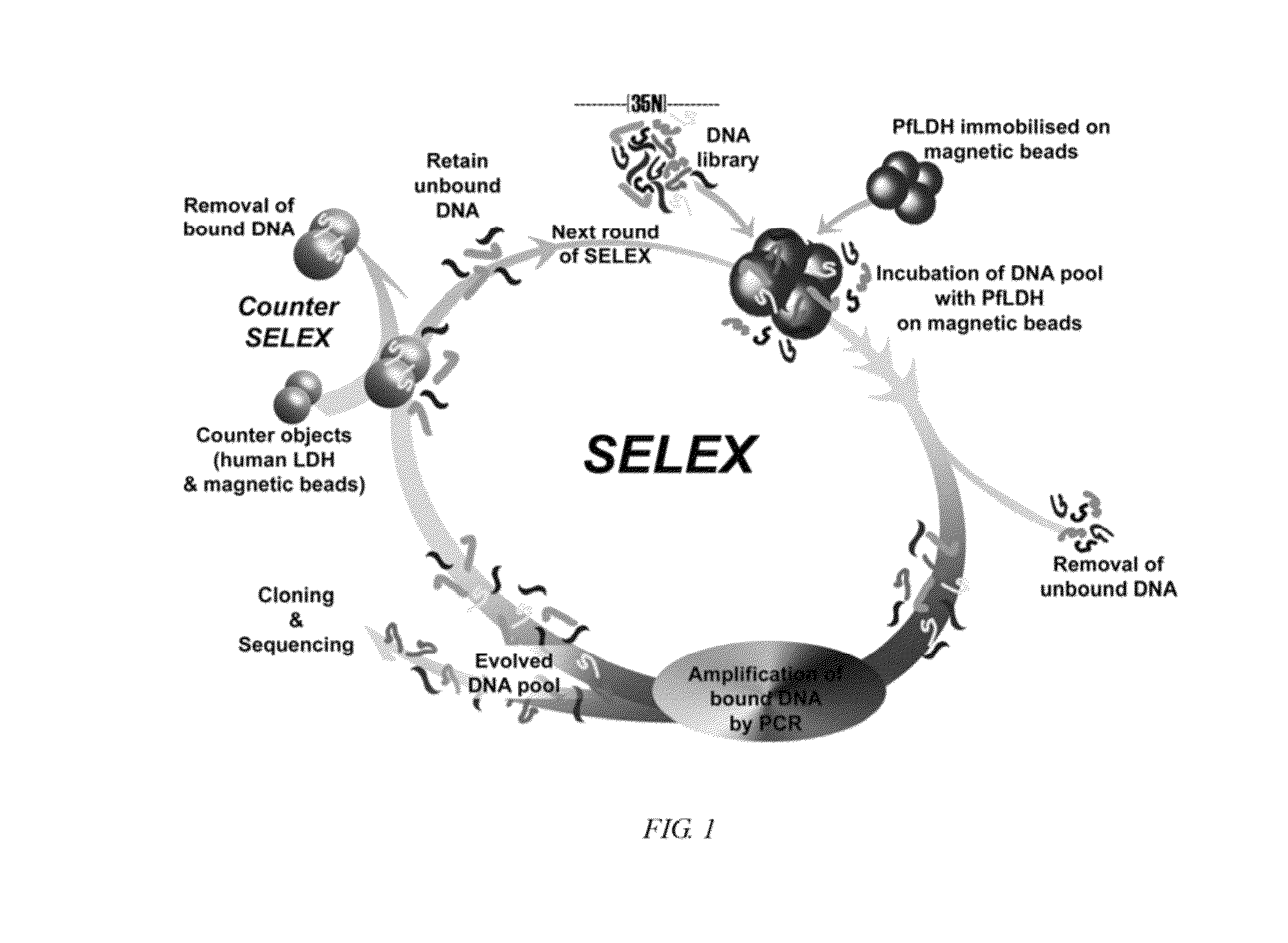
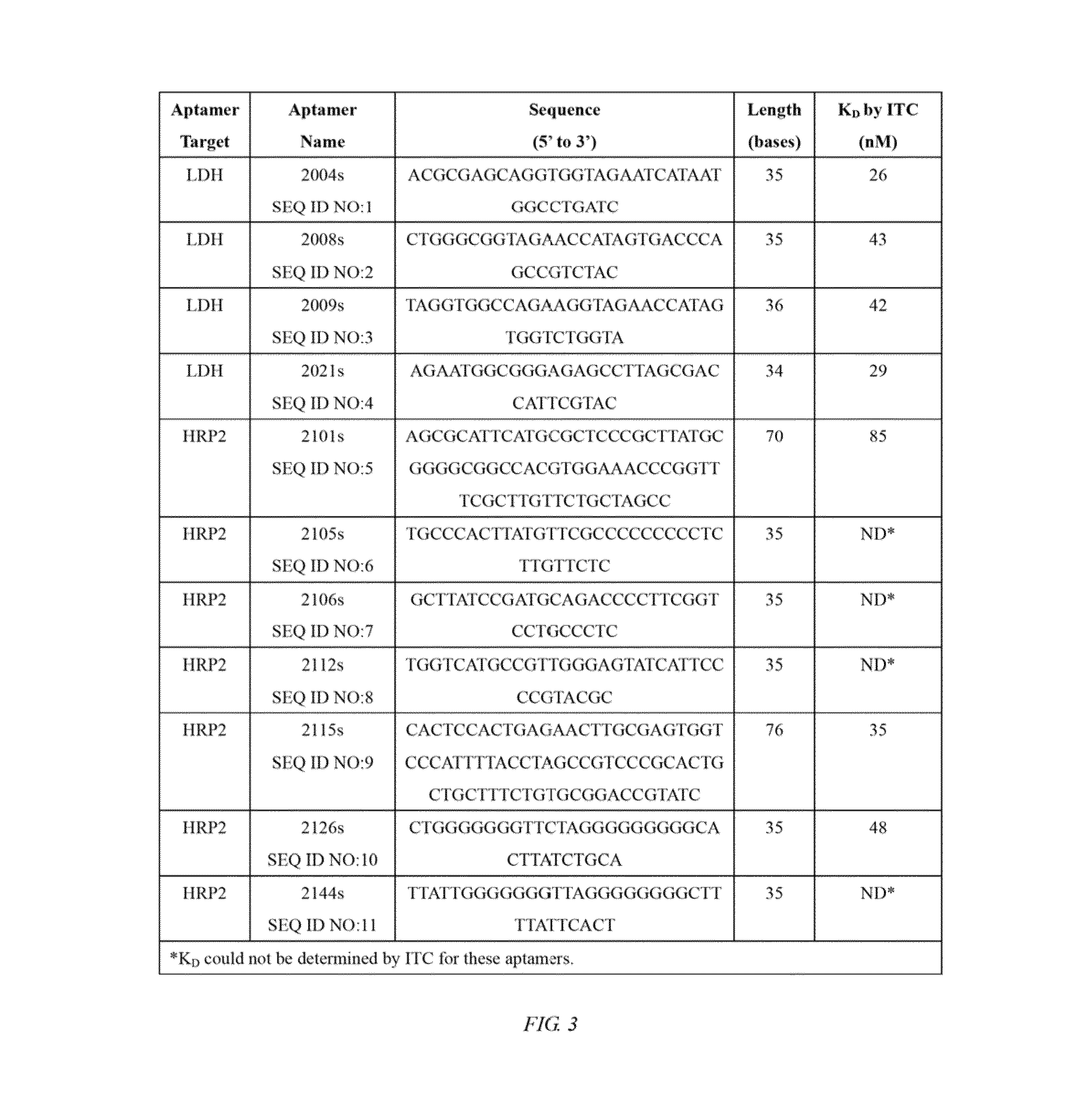
Nucleic acid aptamers against plasmodium lactate dehydrogenase and histidine-rich protein ii and uses thereof for malaria diagnosis
ActiveUS20130210023A1Inhibit functionSugar derivativesMicrobiological testing/measurementAptamerLactate dehydrogenase
The present invention provides nucleic acid aptamers that bind to Plasmodium proteins lactate dehydrogenase and histidine-rich protein II, and uses thereof for the diagnosis of malaria. Aptamers against histidine-rich protein II may be used to detect the presence of Plasmodium species in general, whereas aptamers against lactate dehydrogenase can be used to specifically detect Plasmodium falciparum.
Owner:VERSITECH LTD
Sub-region of a plasmodium protein with improved vaccine potential, and medical uses thereof
The present application relates to a sub-region of a Plasmodium protein, with improved vaccine potential, and to medical uses thereof, notably for treatment or diagnosis of malaria. The present invention notably provides unstructured or unfolded polypeptides deriving from the PFF0165c protein of P. falciparum 3D7. The polypeptides of the invention have a high antigenicity, a high immunogenicity, have a high parasite-killing activity in the ADCI assay, and are strongly associated with clinical protection against malaria, and. The present invention thereby provides a vaccine for the palliative and / or curative treatment of malaria, which is specifically intended for infants, toddlers, children under the age of 5, pregnant women.
Owner:UNIVERSITY OF LAUSANNE +1
DNA APTAMER SPECIFICALLY BINDING TO pLDH (PLASMODIUM LACTATE DEHYDROGENASE)
ActiveUS20120316325A1High sensitivityImprove accuracyOrganic active ingredientsSugar derivativesLactate dehydrogenaseDiagnosis of malaria
Disclosed herein are a DNA aptamer specifically binding to pLDH (Plasmodium Lactate Dehydrogenase), a composition for the diagnosis of malaria, comprising the same, and a diagnostic kit for malaria using the same. Superior in specificity and stability to antibodies which are conventionally used to diagnose malaria, the DNA aptamers specifically binding to pLDH (Plasmodium Lactate Dehydrogenase) in accordance with the present invention can be developed into biosensors which determine pLDH levels with high sensitivity and accuracy, greatly contributing to the diagnostic accuracy of malaria.
Owner:MD APTUS INC
Test strip for rapidly detecting plasmodia based on colloidal gold immunochromatographic assay, as well as antibodies and cell lines thereof
InactiveCN102229913AEasy to operateRapid diagnosisMicroorganism based processesTissue cultureCellulosePolyester
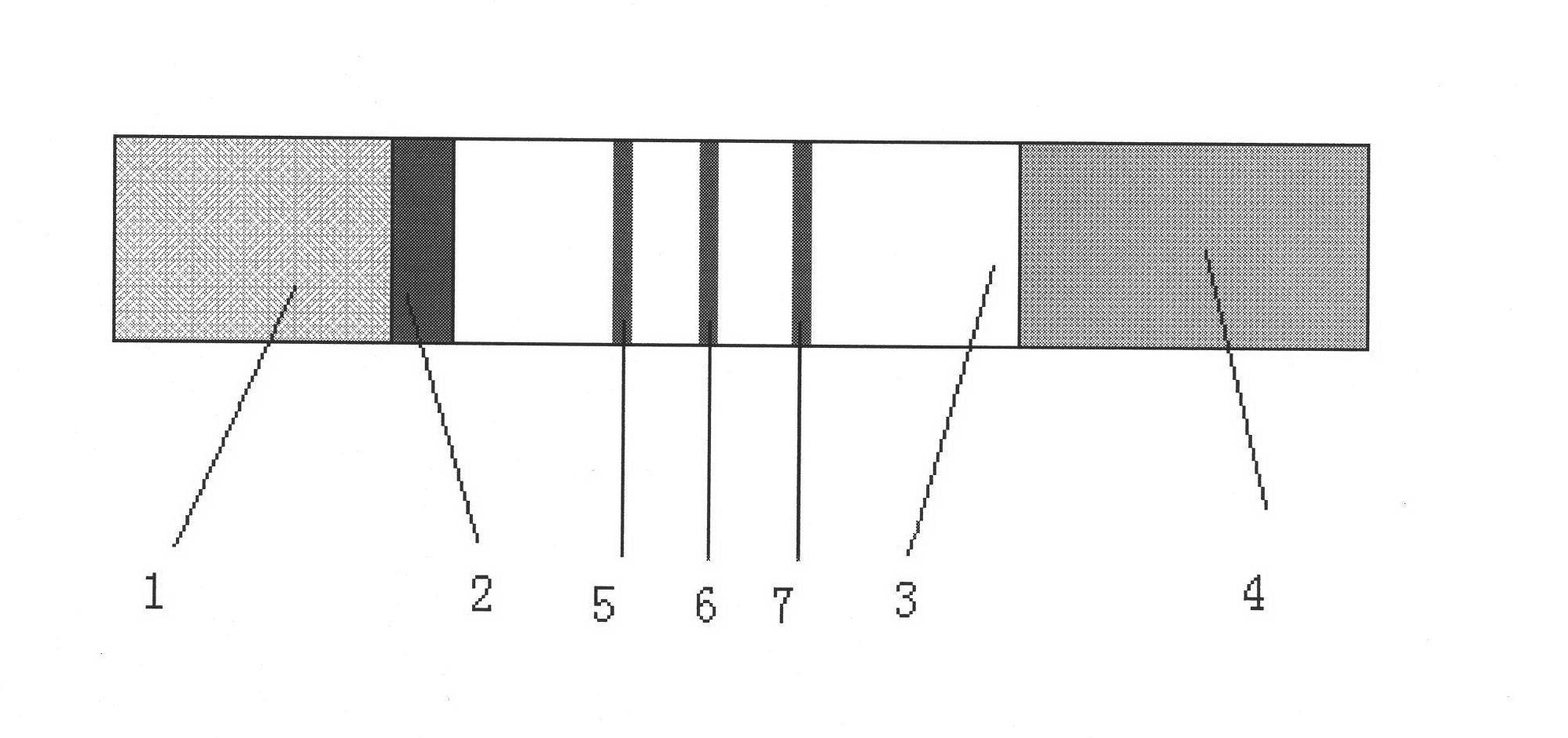
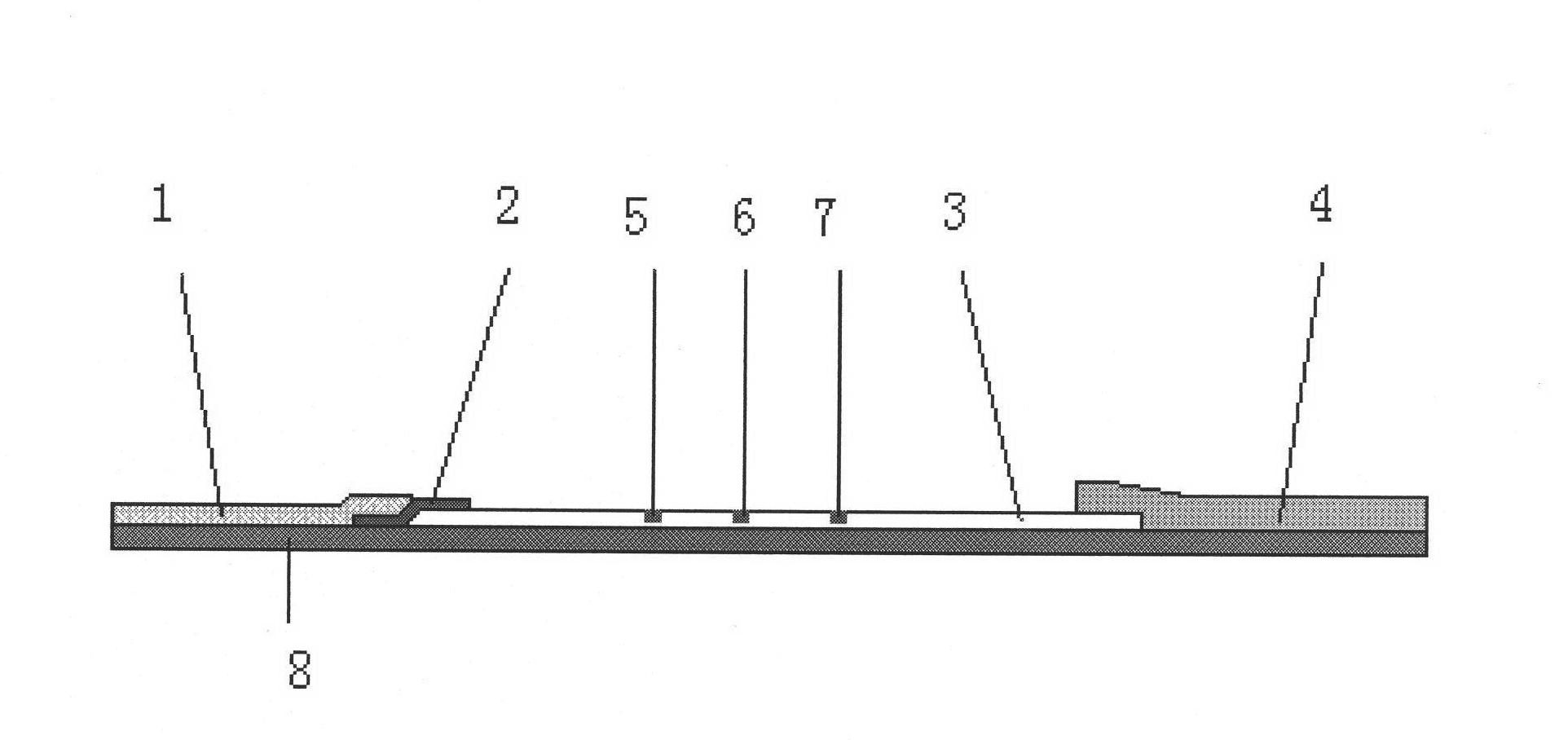
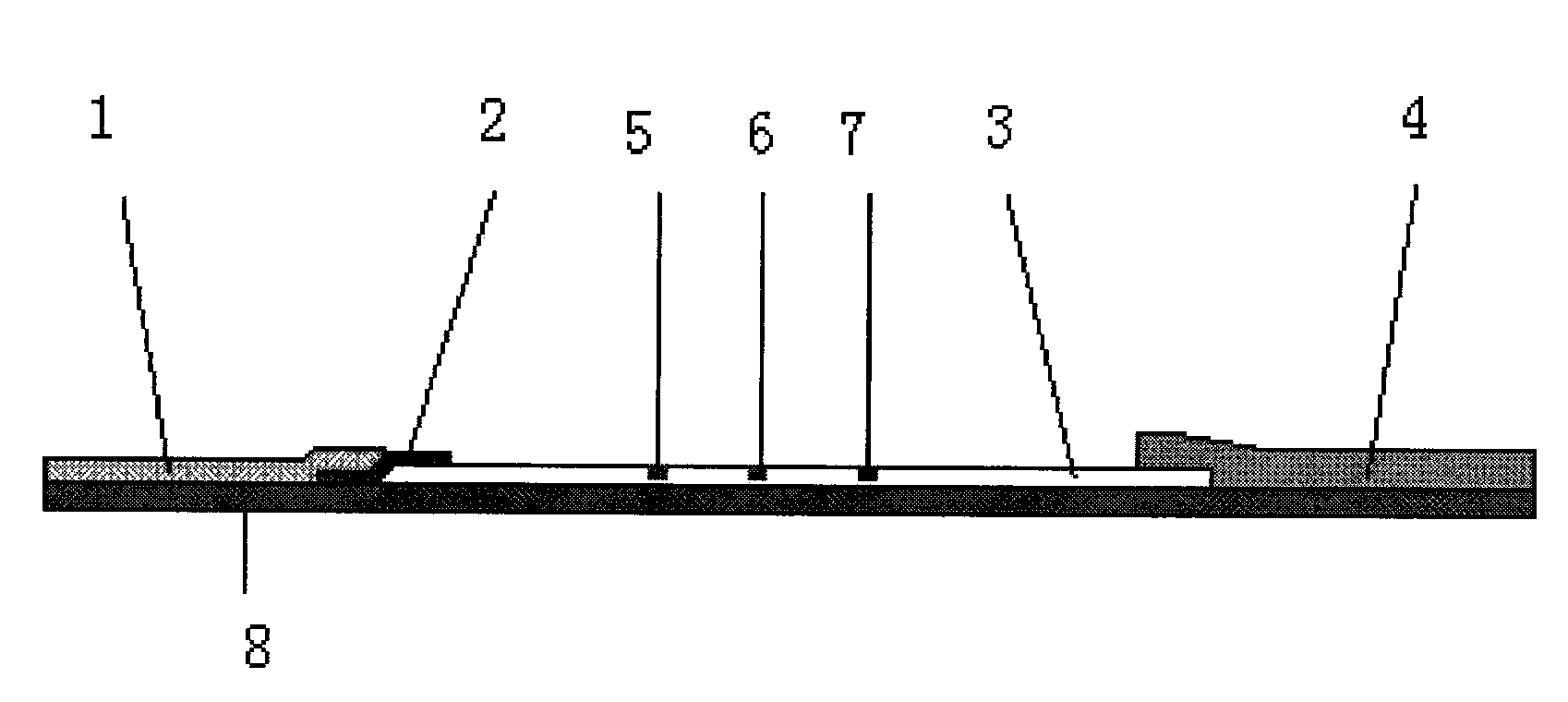
The invention discloses a test strip for rapidly detecting plasmodia based on colloidal gold immunochromatographic assay, as well as antibodies and cell lines thereof. The test strip comprises a basal plate, a sample pad, a polyester cellulose pad, a nitrocellulose membrane and a water absorbent pad, wherein the sample pad, the polyester cellulose pad, the nitrocellulose membrane and the water absorbent pad are partially superposed in sequence along the length direction of the basal plate and fixed on the basal plate; a colloidal gold-antibody I (H12) conjugate coating is sprayed on the polyester cellulose pad; a first test line, a second test line and a quality control line are distributed on the nitrocellulose membrane; the first test line is formed by performing spray-coating on an antibody II (A5) on the nitrocellulose membrane; the second test line is formed by performing spray-coating on an antibody IV (F6) on the nitrocellulose membrane; and the quality control line is formed by performing spray-coating on an goat anti-mouse IgG1 antibody on the nitrocellulose membrane. The test strip provided by the invention is easy to operate, rapid, sensitive and specific, and is applied to the rapid and early-stage diagnosis of malaria.
Owner:SOUTHERN MEDICAL UNIVERSITY
Detection of plasmodium falciparum histidine-rich protein II in saliva malaria patients
InactiveUS20100279319A1Reduce compliance problemCost-effectiveBiological testingAgainst vector-borne diseasesDiagnosis of malariaNon invasive
The detection of PfHRP II in saliva offers a practical, cost-effective alternative to PfHRP II detection in blood as a means for diagnosis of malaria. Collection of saliva is non-invasive, simple, safe, stress free, painless and can be accomplished in primitive settings. The use of Malaria Antigen ELISA kits (CELISA, Cellabs, Australia) used in accord with known procedures for testing blood samples.
Owner:MOREHOUSE SCHOOL OF MEDICINE
Polypeptide, detection device containing polypeptide, and detection kit containing polypeptide
InactiveCN103965321ASatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
The present invention relates to a polypeptide, a detection device containing the polypeptide, and a detection kit containing the polypeptide, wherein the polypeptide comprises the amino acid sequence represented by SEQ ID NO:1. The polypeptide, the detection device containing the polypeptide, and the detection kit containing the polypeptide are applicable to malaria diagnosis.
Owner:苏州工业园区强东医药科技有限公司
Polypeptide, detection device containing polypeptide, and detection kit containing polypeptide
InactiveCN103965311ASatisfactory effectSatisfied with the effectBiological material analysisMicroorganism based processesDiagnosis of malariaMalaria
The present invention relates to a polypeptide, a detection device containing the polypeptide, and a detection kit containing the polypeptide, wherein the polypeptide comprises the amino acid sequence represented by SEQ ID NO:1. The polypeptide, the detection device containing the polypeptide, and the detection kit containing the polypeptide provide applications in malaria diagnosis.
Owner:SUZHOU SJ BIOMATERIALS
Polypeptide, detection device containing polypeptide, and detection kit containing polypeptide
InactiveCN103965333ASatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
Owner:SUZHOU SJ BIOMATERIALS
Polypeptide as well as detection device and detection reagent kit comprising polypeptide
InactiveCN103965323ASatisfactory effectSatisfied with the effectBiological material analysisMicroorganism based processesDiagnosis of malariaMalaria
The invention relates to a polypeptide as well as a detection device and a detection reagent kit comprising the polypeptide. The polypeptide provided by the invention consists of an amino acid sequence shown by SEQ ID NO:1. The polypeptide as well as the detection device and the detection reagent kit comprising the polypeptide are useful in the diagnosis of malaria.
Owner:苏州工业园区强东医药科技有限公司
DNA aptamer specifically binding to pLDH (plasmodium lactate dehydrogenase)
ActiveUS8541561B2Improve diagnostic accuracyStrong specificityOrganic active ingredientsSugar derivativesLactate dehydrogenaseDiagnosis of malaria
Disclosed herein are a DNA aptamer specifically binding to pLDH (Plasmodium Lactate Dehydrogenase), a composition for the diagnosis of malaria, comprising the same, and a diagnostic kit for malaria using the same. Superior in specificity and stability to antibodies which are conventionally used to diagnose malaria, the DNA aptamers specifically binding to pLDH (Plasmodium Lactate Dehydrogenase) in accordance with the present invention can be developed into biosensors which determine pLDH levels with high sensitivity and accuracy, greatly contributing to the diagnostic accuracy of malaria.
Owner:MD APTUS INC
Polypeptide, detection device containing polypeptide, and detection kit containing polypeptide
InactiveCN103965312ASatisfactory effectSatisfied with the effectBiological material analysisMicroorganism based processesDiagnosis of malariaMalaria
The present invention relates to a polypeptide, a detection device containing the polypeptide, and a detection kit containing the polypeptide, wherein the polypeptide comprises the amino acid sequence represented by SEQ ID NO:1. The polypeptide, the detection device containing the polypeptide, and the detection kit containing the polypeptide provide applications in malaria diagnosis.
Owner:苏州工业园区强东医药科技有限公司
Polypeptide as well as detection device and detection reagent kit comprising polypeptide
InactiveCN103965324ASatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
The invention relates to a polypeptide as well as a detection device and a detection reagent kit comprising the polypeptide. The polypeptide provided by the invention consists of an amino acid sequence shown by SEQIDNO:1. The polypeptide as well as the detection device and the detection reagent kit comprising the polypeptide are useful in the diagnosis of malaria.
Owner:苏州工业园区强东医药科技有限公司
Nucleic acid aptamers against plasmodium lactate dehydrogenase and histidine-rich protein II and uses thereof for malaria diagnosis
ActiveUS9000137B2Sugar derivativesMicrobiological testing/measurementLactate dehydrogenaseDiagnosis of malaria
The present invention provides nucleic acid aptamers that bind to Plasmodium proteins lactate dehydrogenase and histidine-rich protein II, and uses thereof for the diagnosis of malaria. Aptamers against histidine-rich protein II may be used to detect the presence of Plasmodium species in general, whereas aptamers against lactate dehydrogenase can be used to specifically detect Plasmodium falciparum.
Owner:VERSITECH LTD
Polypeptide, detection device containing polypeptide, and detection kit containing polypeptide
InactiveCN103965313ASatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
The present invention relates to a polypeptide, a detection device containing the polypeptide, and a detection kit containing the polypeptide, wherein the polypeptide comprises the amino acid sequence represented by SEQ ID NO:1. The polypeptide, the detection device containing the polypeptide, and the detection kit containing the polypeptide provide applications in malaria diagnosis.
Owner:苏州工业园区强东医药科技有限公司
Polypeptide, detection device and detection kit containing the polypeptide
InactiveCN103965319ASatisfactory effectSatisfied with the effectBiological material analysisMicroorganism based processesDiagnosis of malariaMalaria
The invention relates to polypeptide, a detection device and a detection kit containing the polypeptide. The polypeptide is formed by an amino acid sequence shown in SEQ ID NO: 1. The polypeptide, the detection device, and the detection kit are useful in diagnosis of malaria.
Owner:苏州工业园区强东医药科技有限公司
Sub-region of a plasmodium protein with improved vaccine potential, and medical uses thereof
The present application relates to a sub-region of a Plasmodium protein, with improved vaccine potential, and to medical uses thereof, notably for treatment or diagnosis of malaria. The present invention notably provides unstructured or unfolded polypeptides deriving from the PFF0165c protein of P. falciparum 3D7. The polypeptides of the invention have a high anti-genicity, a high immunogenicity, have a high parasite-killing activity in the ADCI assay, and are strongly associated with clinical protection against malaria, and. The present invention thereby provides a vaccine for the palliative and / or curative treatment of malaria, which is specifically intended for infants, toddlers, children under the age of 5, pregnant women.
Owner:UNIVERSITY OF LAUSANNE +1
Polypeptide, detection device containing polypeptide, and detection kit containing polypeptide
InactiveCN105646691ASatisfactory effectSatisfied with the effectDepsipeptidesImmunoglobulinsDiagnosis of malariaMalaria
The present invention relates to a polypeptide, a detection device containing the polypeptide, and a detection kit containing the polypeptide, wherein the polypeptide comprises the amino acid sequence represented by SEQ ID NO:1. According to the present invention, the polypeptide, the detection device containing the polypeptide, and the detection kit containing the polypeptide provide effects in diagnosis of malaria.
Owner:THE FIRST AFFILIATED HOSPITAL OF SOOCHOW UNIV
Polypeptide, detection device and detection kit containing the polypeptide
InactiveCN103965320ASatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
The invention relates to polypeptide, a detection device and a detection kit containing the polypeptide. The polypeptide is formed by an amino acid sequence shown in SEQ ID NO: 1. The polypeptide, the detection device, and the detection kit are useful in diagnosis of malaria.
Owner:SUZHOU SJ BIOMATERIALS
Polypeptide, and a detection device and a detection kit containing polypeptide
InactiveCN105646687ASatisfactory effectSatisfied with the effectDepsipeptidesImmunoglobulinsDiagnosis of malariaMalaria
The invention relates to a polypeptide, and a detection device and a detection kit containing the polypeptide. The polypeptide provided by the invention is composed of an amino acid sequence as shown in SEQ ID NO: 1. The polypeptide, and the detection device and the detection kit containing the polypeptide provided by the invention are useful in diagnosis of malaria.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Polypeptide and detection device and detection kit containing polypeptide
InactiveCN105646689ASatisfactory effectSatisfied with the effectDepsipeptidesImmunoglobulinsDiagnosis of malariaMalaria
The invention relates to a polypeptide and a detection device and detection kit containing the polypeptide. The polypeptide disclosed by the invention is made up of an amino acid sequence represented by SEQ ID NO: 1 shown in the description. The polypeptide and the detection device and detection kit containing the polypeptide, disclosed by the invention, are useful in malaria diagnosis.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Polypeptide, detection device and detection kit comprising the same
InactiveCN103965334BSatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaAmino acid
The present invention relates to a polypeptide, a detection device and a detection kit comprising the polypeptide. The polypeptide of the present invention consists of SEQ? ID? NO: The amino acid sequence shown in 1 constitutes. The polypeptide, detection device and detection kit comprising the polypeptide of the present invention are useful in the diagnosis of malaria.
Owner:苏州工业园区强东医药科技有限公司
Kits that can be used to aid in the diagnosis of malaria
The invention relates to a reagent kit and a diagnostic method thereof which can be used for auxiliary diagnosis of malaria. The kit of the present invention includes one or more solid supports, and a specific set of polypeptides independently linked to the one or more solid supports. The product of the invention can be used for auxiliary detection of malaria, especially Plasmodium falciparum infection.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Polypeptide, detection device and detection kit comprising the polypeptide
InactiveCN103965313BSatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
The present invention relates to polypeptides, detection devices and detection kits comprising the polypeptides. The polypeptide of the present invention consists of SEQ? ID? The amino acid sequence represented by NO: 1 is constituted. The polypeptides of the present invention, detection devices and detection kits comprising the polypeptides are useful in the diagnosis of malaria.
Owner:苏州工业园区强东医药科技有限公司
Polypeptide as well as detection device and detection reagent kit comprising polypeptide
InactiveCN103965317ASatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
The invention relates to a polypeptide as well as a detection device and a detection reagent kit comprising the polypeptide. The polypeptide provided by the invention consists of an amino acid sequence shown by SEQ ID NO:1. The polypeptide as well as the detection device and the detection reagent kit comprising the polypeptide are useful in the diagnosis of malaria.
Owner:苏州工业园区强东医药科技有限公司
Polypeptide used for auxiliary diagnosis of malaria, detection member comprising polypeptide and detection kit
InactiveCN105646693ASatisfactory effectSatisfied with the effectDepsipeptidesImmunoglobulinsDiagnosis of malariaAIDS diagnosis
The present invention relates to a polypeptide used for auxiliary diagnosis of malaria, a detection member comprising the polypeptide and a detection kit. The polypeptide comprises an amino acid sequence shown in SEQIDNO: 1. The polypeptide, the detection member containing the polypeptide, and the detection kit can be used in the auxiliary diagnosis of malaria.
Owner:302 MILITARY HOSPITAL OF CHINA
Immunity-chromatography kit for rapid diagnosis of malaria and its pathogen species and preparation method thereof
ActiveCN102103141BIncreased sensitivityStrong specificityTissue cultureImmunoglobulins against enzymesMalarial parasiteCellulose
The invention discloses an immunity-chromatography kit for the rapid diagnosis of malaria and its pathogen species and a preparation method thereof. The kit comprises a colloidal gold immunity-chromatography test strip, a matching cell-free lysate, a sample cup, a blood taking needle, and an operating instruction. The colloidal gold immunity-chromatography test strip comprises a sample pad, a colloidal-gold pad, a cellulose membrane, and a water-absorbing pad, wherein the colloidal-gold pad contains colloidal gold labelled antibodies; a detection line and a quality control line are disposed on the cellulose membrane; and the colloidal gold labelled antibody and the detection line are composed of monoclonal antibodies which can specifically bind the lactate dehydrogenase of plasmodia. The immunity-chromatography kit for the rapid diagnosis of malaria and its pathogen of the invention can distinguish falciparum malaria from vivax malaria, has the advantages of simplicity, sensitivity, specificity, and rapidity, and is suitable for clinical and field applications.
Owner:SHANGHAI NEW JIEER CLEANING PRODS
Polypeptide, detection device and detection kit comprising the polypeptide
InactiveCN103965318BSatisfactory effectSatisfied with the effectBiological material analysisMicroorganism based processesDiagnosis of malariaAmino acid
The present invention relates to a polypeptide, a detection device and a detection kit comprising the polypeptide. The polypeptide of the present invention consists of the amino acid sequence shown in SEQ ID NO:1. The polypeptide, detection device and detection kit comprising the polypeptide of the present invention are useful in the diagnosis of malaria.
Owner:苏州工业园区强东医药科技有限公司
Polypeptide, detection device containing same and detection kit
InactiveCN103965339ASatisfactory effectSatisfied with the effectBiological material analysisDepsipeptidesDiagnosis of malariaMalaria
The invention relates to a polypeptide, a detection device containing the same and a detection kit. The polypeptide is composed of the amino acid sequence shown as SEQ ID NO: 1. The polypeptide, the detection device containing the same and the detection kit are useful for diagnosis on malaria.
Owner:苏州工业园区强东医药科技有限公司
Polypeptide, detective device and detective kit containing the polypeptide
InactiveCN103965326ASatisfactory effectSatisfied with the effectBiological material analysisMicroorganism based processesDiagnosis of malariaMalaria
The present invention relates to a polypeptide, a detective device and a detective kit containing the polypeptide. The polypeptide consists of an amino acid sequences represented by SEQ ID NO:1. According to the invention, the polypeptide, the detective device and the detective kit containing the polypeptide are useful in the diagnosis of malaria.
Owner:SUZHOU SJ BIOMATERIALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com