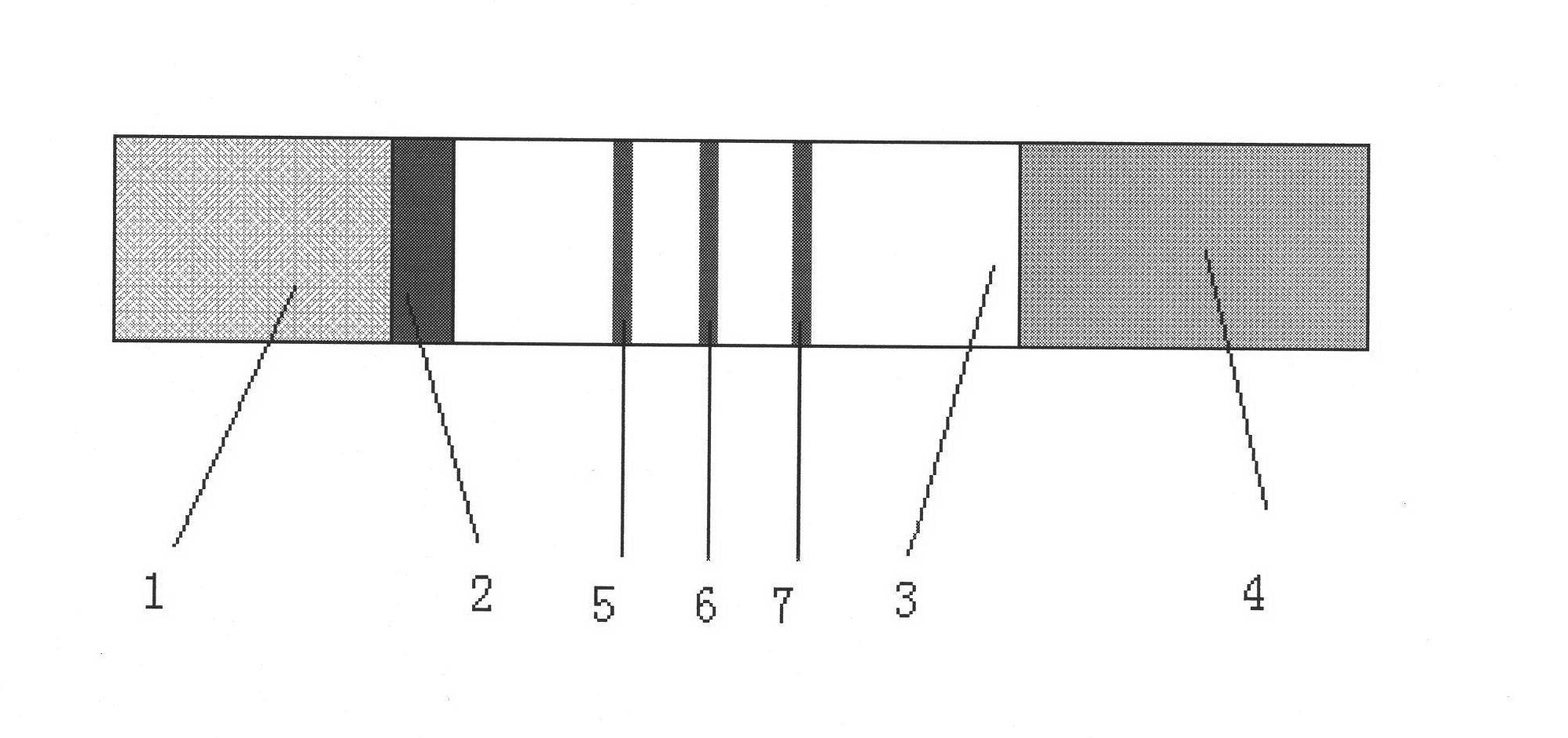
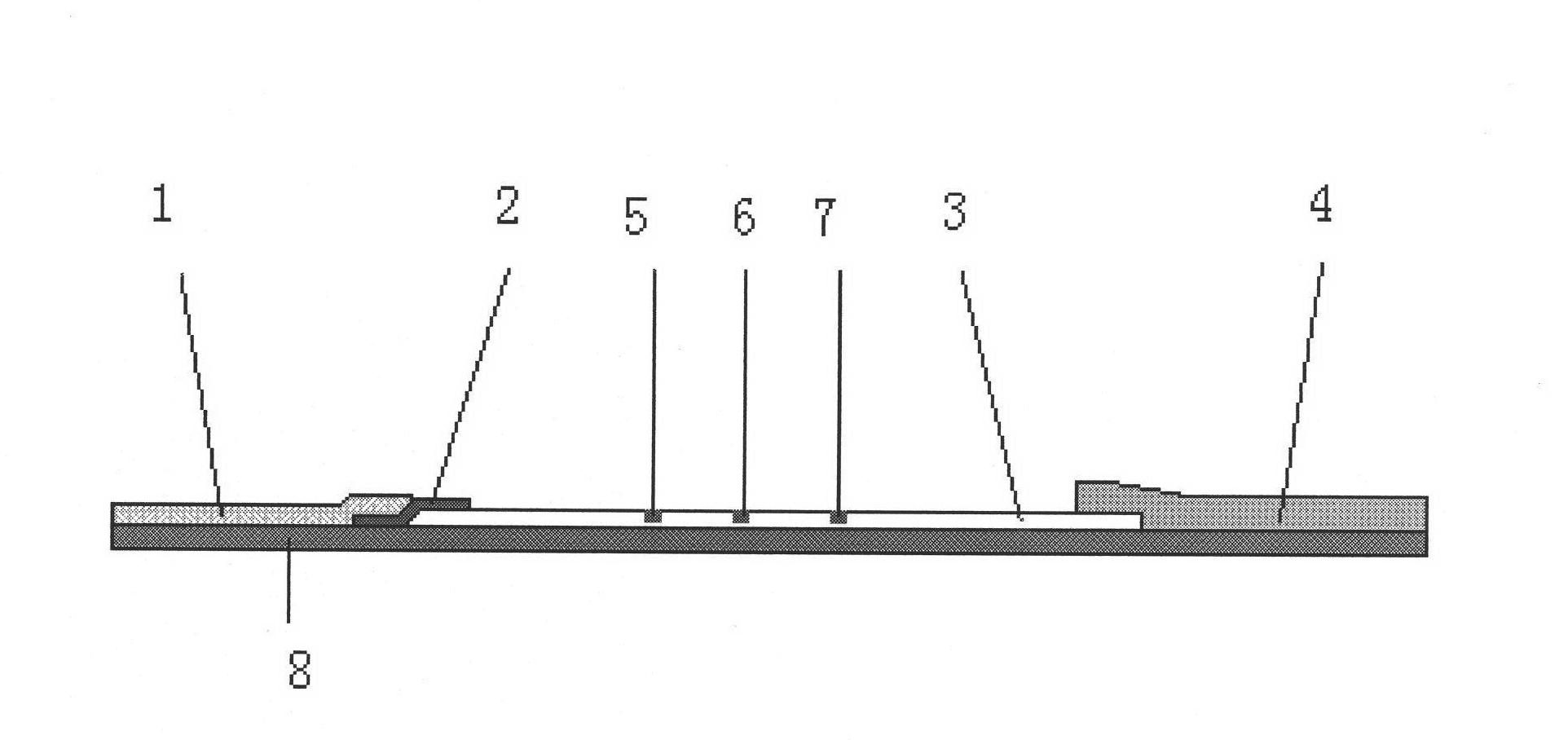
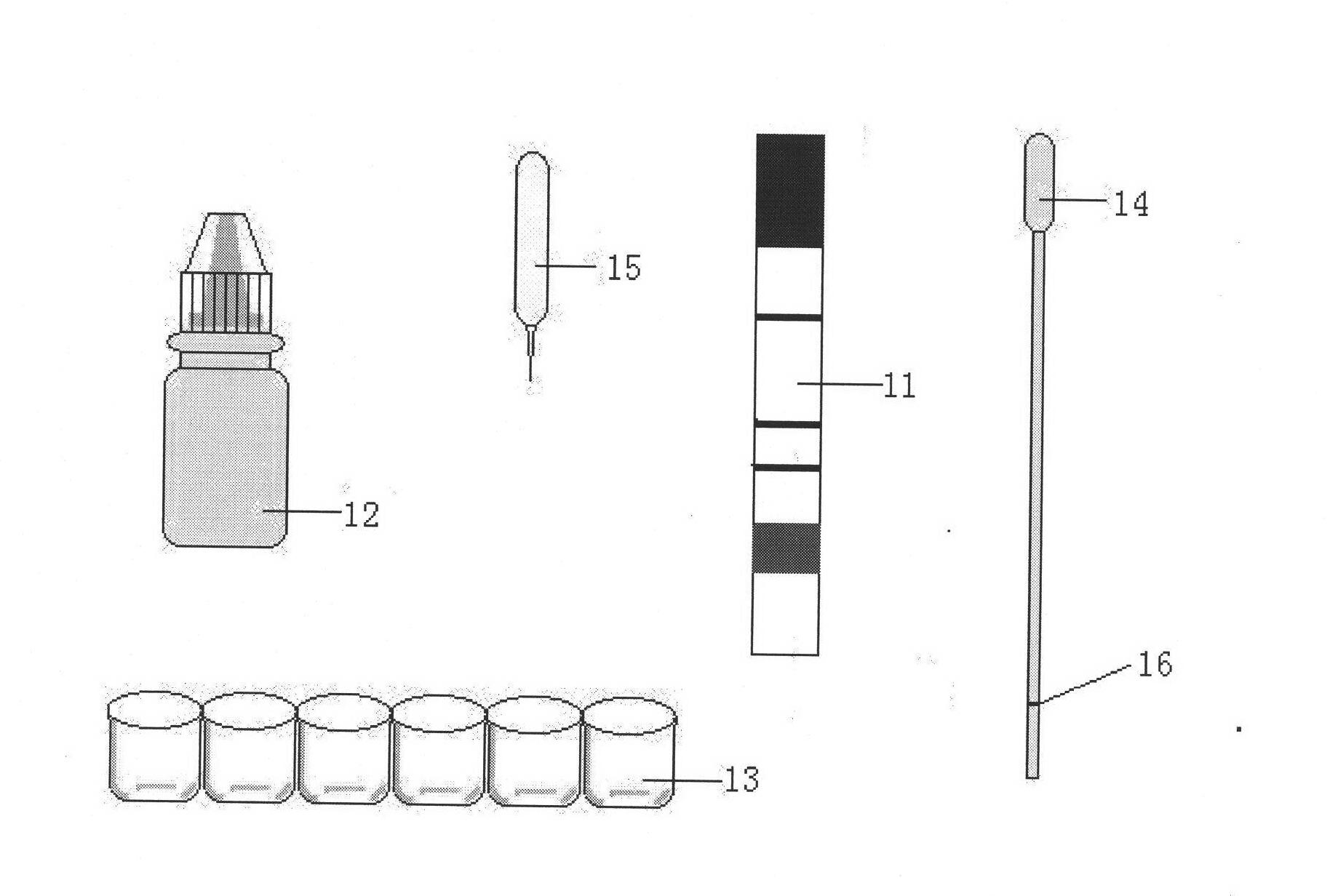
Immunity-chromatography kit for rapid diagnosis of malaria and its pathogen species and preparation method thereof
A technology of immune chromatography and pathogens, applied in the field of disease detection, can solve problems such as not suitable for large-scale field application, limited practical value of disease control work, complex equipment and instruments, etc., achieve rapid and intuitive result observation, simple and fast detection method, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1. Preparation of Plasmodium Antigens
[0065] (1) PCR amplification of pLDH gene primers
[0066] According to BzikDJ, FoxBA, GonyerK. Expression of Plasmodium falciparumlactatedehydrogenasein Escherichiacoli [J]. MolBiochemParasitol, 199359: 155-166. Design a pair of primers for amplifying the full-length coding gene of Plasmodium falciparum LDH.
[0067] P1: 5′ ATGGCACCAAAAGCAAAAATCGT 3′
[0068] P2: 5′TTAAGCTAATGCCTTCATTCCTCT 3′
[0069] (2) template
[0070] Heparin anticoagulated whole blood collected from patients with falciparum malaria in Yunnan was treated as a template for PCR amplification according to the method of John E. Protocols inmolecular parasitology [M]. , 0.015% saponin, 1mmol / L ethylenediaminetetraacetic acid (EDTA)] shake and mix well, place at room temperature at 10mim to fully dissolve red blood cells, centrifuge at 10000×g for 10min at room temperature, and then use 250μL buffer [10mmol / L trimethylol Aminomethane (Tris)-HCl, pH 8.3...
Embodiment 2
[0073] Example 2. Preparation of Plasmodium Lactate Dehydrogenase Monoclonal Antibody
[0074] Immunize each BALB / c mouse with the first intraperitoneal injection of 100 μg of the above-mentioned purified recombinant fusion protein + Freund’s complete adjuvant suspension, and then inject 100 μg of the purified recombinant fusion protein + Freund’s incomplete adjuvant mixture every 1 month. Suspension once, a total of 2 times, and 3 days before the spleen was killed for cell fusion, the antigen was directly injected through the tail vein to boost the immunization once.
[0075] Production and screening of hybridoma cells The fusion of SP2 / 0 tumor cells and splenocytes of immune mice and the cloning of hybridoma cells were carried out according to the routine methods of our laboratory. Use the above-mentioned purified recombinant fusion protein and glutathione 2S2 transferase (GST) protein to coat the plate respectively, and perform conventional ELISA to detect antibody secretio...
Embodiment 3
[0086] Example 3. Preparation of Plasmodium Specific Antibody Gold Probe Solution
[0087] Prepare colloidal gold probe solution and gold-labeled antibody pad labeled with Plasmodium-specific antibody as follows: Colloidal gold is prepared by citric acid reduction method: HAuCl with a mass percentage concentration of 0.01% 4 (Shanghai trial brand was purchased from Shanghai Sinopharm Group Chemical Reagent Co., Ltd.) aqueous solution was boiled, and 2 mL of trisodium citrate aqueous solution with a mass percentage concentration of 1% was added under stirring, and continued boiling until the liquid was dark red to obtain a colloidal gold solution.
[0088] Determination of colloidal gold-conjugated antibody saturation: use 0.1M K 2 CO 3 Adjust the pH value of the solution to 8.5, prepare a 96-well microtiter plate, dilute the Plasmodium-specific antibody with 0.05M boric acid buffer in the well, make a dilution gradient, add the same volume of colloidal gold to mix, and then a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com