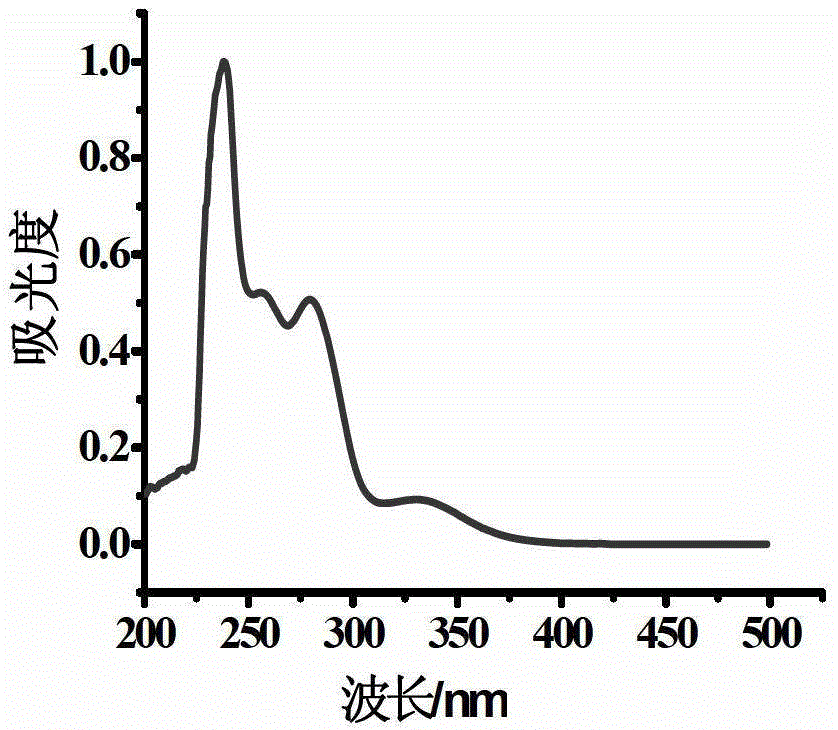
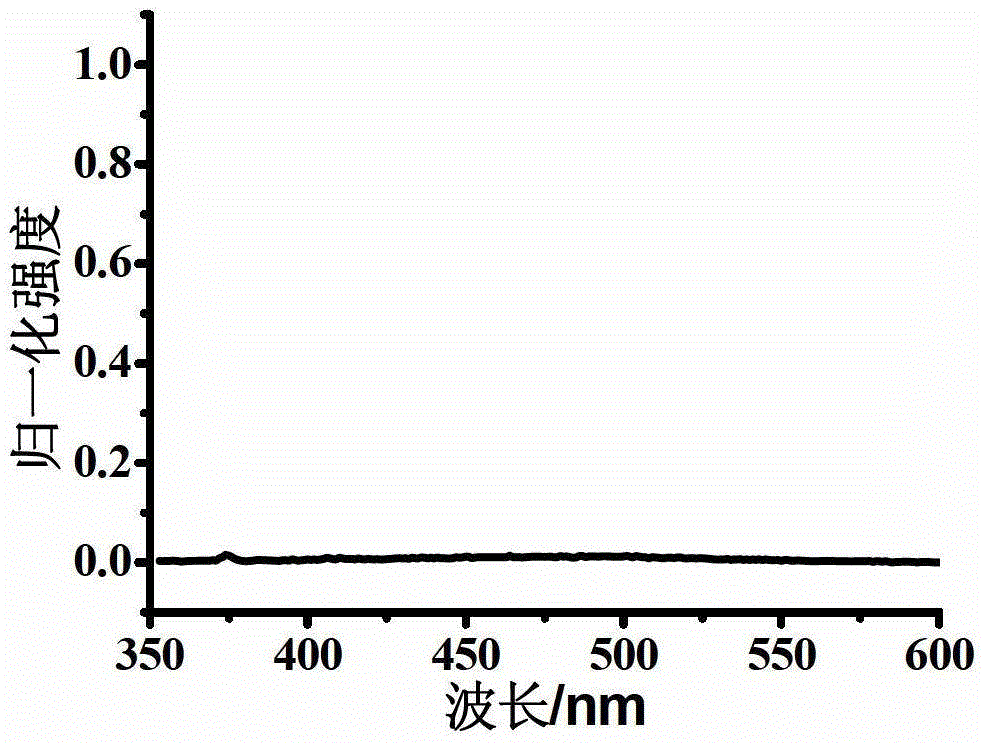
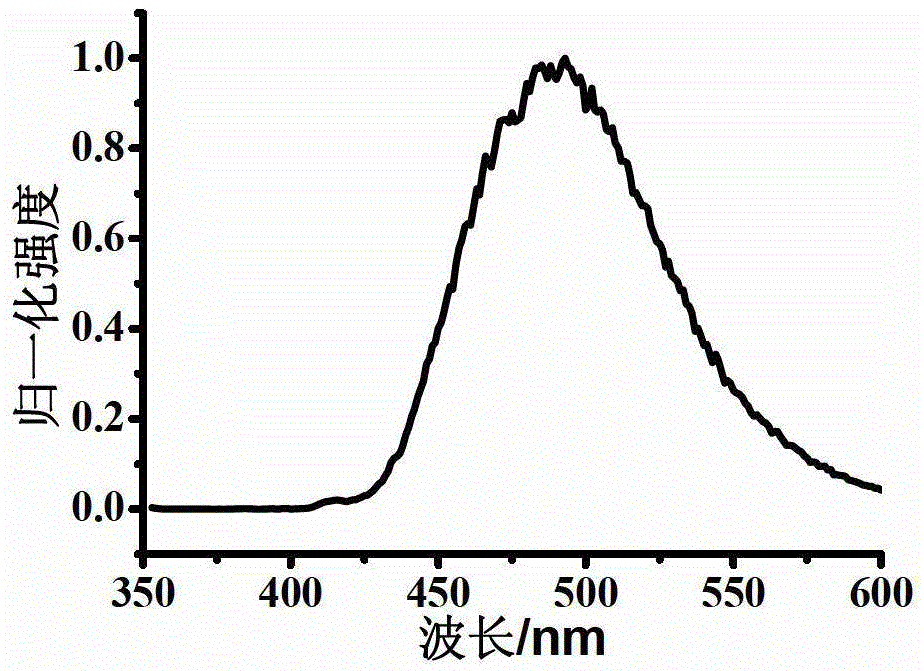
Oxidized thioxanthone derivatives, preparation method and application thereof
A technology for oxidizing thioxanthone and thioxanthone, which is applied in the fields of chemical instruments and methods, organic chemistry, semiconductor/solid-state device manufacturing, etc., and can solve the problem that there is no host material for the light-emitting layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Synthesis of Oxythioxanthone Derivatives Comp-1
[0080]
[0081] Dissolve 2,7-diphenylthioxanthone and 1M nitrate solution in acetonitrile at a molar ratio of 1:4, stir at room temperature for 4 hours, add a large amount of water to precipitate, filter and recrystallize with ethanol to obtain Oxidized thioxanthone derivative Comp-1, the yield is about 70%; m / z: 380.09 (100.0%), 381.09 (28.0%), 382.08 (4.6%), 381.08 (4.2%), 382.09 (1.7 %). m / z is the mass-to-charge ratio of the target molecule in a low-resolution mass spectrum.
Embodiment 2
[0083] Synthesis of oxidized thioxanthone derivatives Comp-2:
[0084]
[0085] As in Example 1, 2,7-bis(2'-biphenyl)thioxanthone was used instead of 2,7-diphenylthioxanthone to obtain oxidized thioxanthone derivatives Comp-2, which produced The rate is about 70%; m / z: 532.15 (100.0%), 533.15 (40.8%), 534.16 (8.0%), 533.14 (4.8%).
Embodiment 3
[0087] Synthesis of oxidized thioxanthone derivatives Comp-3:
[0088]
[0089] As in Example 1, 2,7-bis(3',5'-terphenyl)thioxanthone was used instead of 2,7-diphenylthioxanthone to obtain oxidized thioxanthone derivatives Comp- 3. The yield is about 70%. m / z: 684.21 (100.0%), 685.22 (53.3%), 686.23 (14.6%), 685.21 (3.2%), 686.22 (1.9%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com