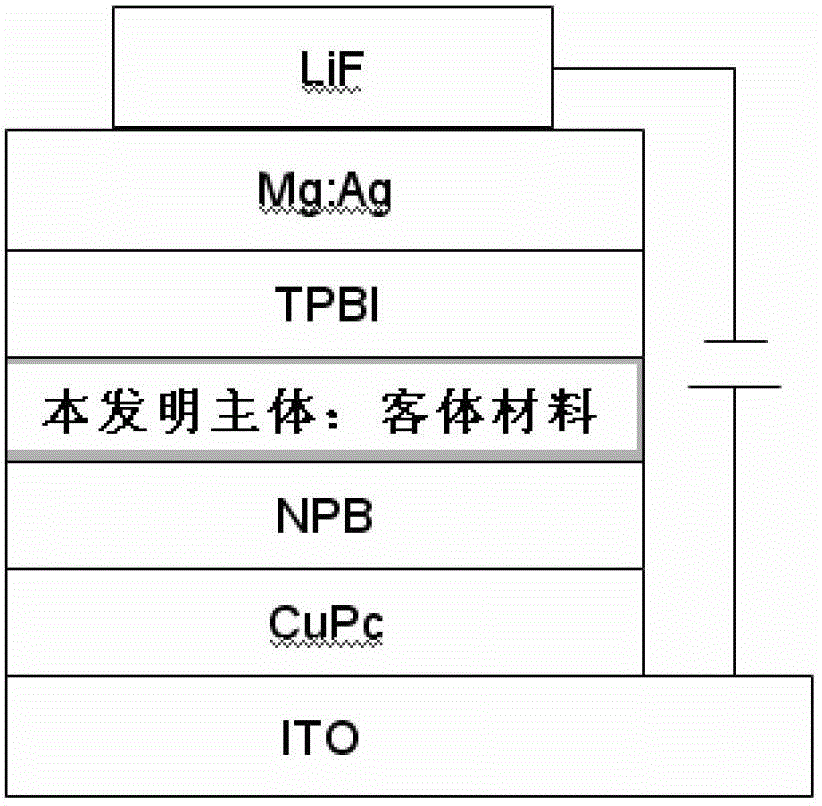
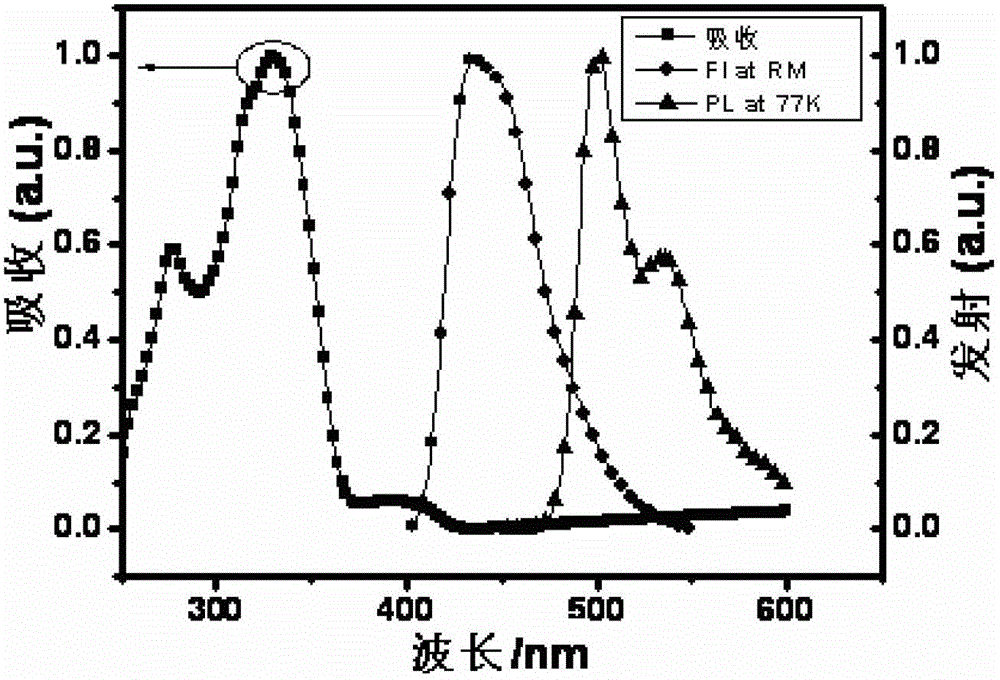
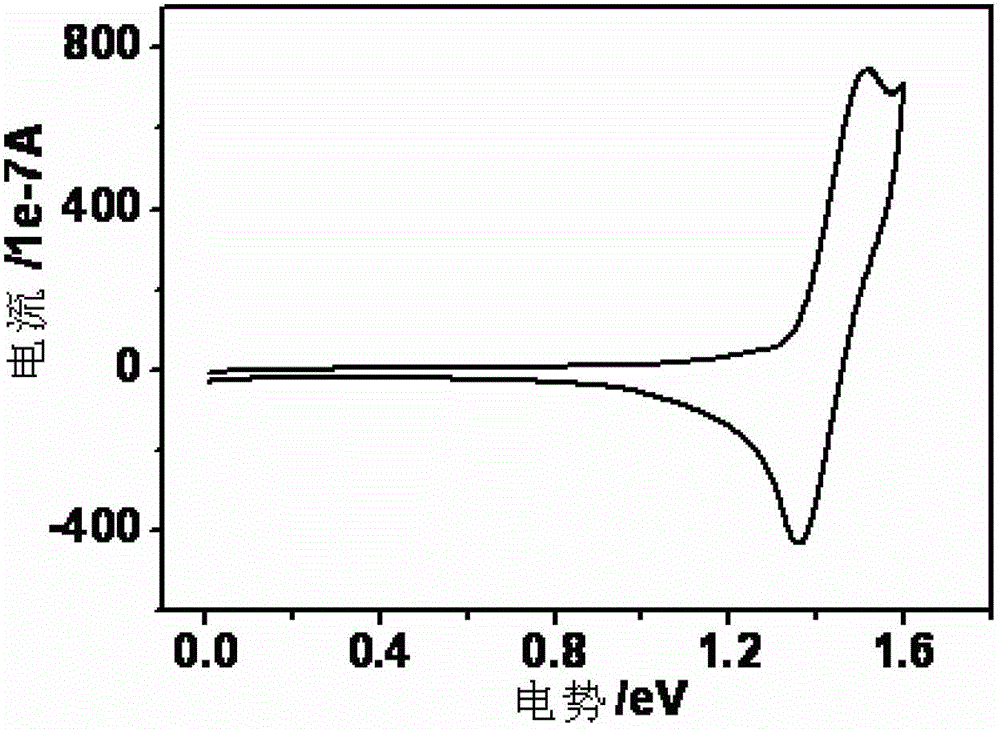
Thioxanthone oxide derivative, preparation method and application thereof
A technology for oxidizing thioxanthone and thioxanthone, which is applied in chemical instruments and methods, silicon organic compounds, compounds of group 4/14 elements of the periodic table, etc., and can solve the problem of no host material for the light-emitting layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Synthesis of oxidized thioxanthone derivatives A-1:
[0120] The synthesis of 2-bromothioxanthone is referenced from Contribution From The Chemical Laboratory Of Iowa State College, vol.24, 1914~1916; William G P, Samuel S. The interaction of aromatic disulphides and sulfuric acid [J], JSC, 1910, 19II: 640-649.
[0121] The synthesis of 1,4-diphenylboronic acid pinacol ester refers to Chem.Eur.J.2004,10,2681–2688; Adv.Funct.Mater.2009,19,277-284; Adv.Funct.Mater.2007,17 ,2432–2438.
[0122] (1) Under the protection of an inert gas, mix 2-bromothioxanthone and 1,4-phenylenediboronic acid penalyl ester at a molar ratio of 2.5:1, and add 0.05 equivalent of tetrakis(triphenyl base phosphine) palladium and 5 equivalents of potassium carbonate, then the mixture was refluxed at 96°C for 8 hours in a mixed solvent of toluene, ethanol, and water with a volume ratio of 4:3:2, and the product was extracted, and the product was subjected to column chromatography (with The volume...
Embodiment 2
[0128] Synthesis of oxidized thioxanthone derivatives A-4:
[0129]
[0130] Same as Example 1, with 3-bromothioxanthone instead of 2-bromothioxanthone, and 1,3-benzenediboronic acid pinacol ester instead of 1,4-benzenediboronic acid pinacol ester, to obtain oxidized Thioxanthone derivative A-4, the yield is about 71%. EI-MS, m / z: 530.06 (100.0%), 531.07 (34.2%), 532.06 (8.4%).
Embodiment 3
[0132] Synthesis of oxidized thioxanthone derivatives A-7:
[0133]
[0134] Same as in Example 1, using 1,1'-biphenyl diboronic acid linalyl ester instead of 1,4-phenylene diboronic acid linalyl ester to obtain oxidized thioxanthone derivative A-7 with a yield of about 70 %. EI-MS, m / z: 606.10 (100.0%), 607.09 (43.1%), 608.11 (9.2%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
| Luminous efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com