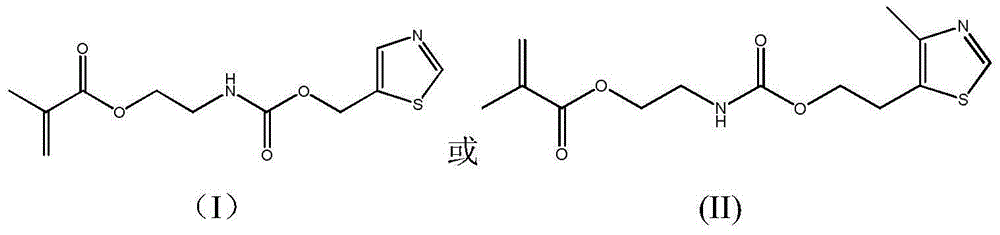
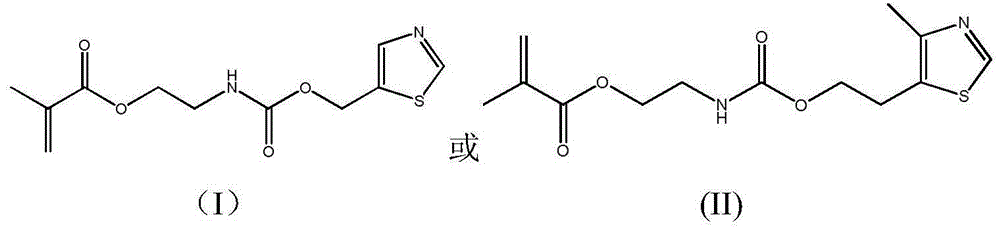
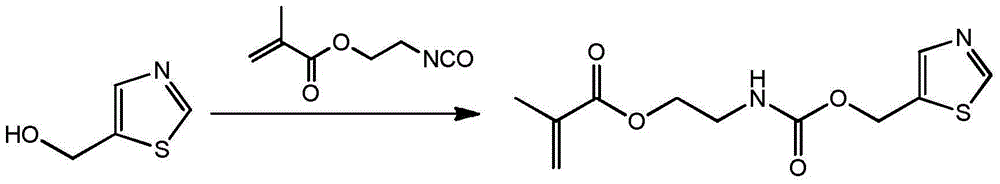
Antibacterial methacrylate monomer containing thiazole ring structure and its preparation method and application
A technology of methacrylate and methacrylic acid, used in dental preparations, dental prostheses, compression mold cups, etc., can solve the problem of lack of antibacterial properties of dental restoration materials, and achieve the effect of broad-spectrum and high-efficiency antibacterial properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) The preparation method of monomer is as follows:
[0028] Add 11.5g 5-hydroxymethylthiazole in the 150ml there-necked flask that magnetic stirring bar is housed, constantly add 15.5g methacrylic acid (2-isocyanatoethyl) ester through constant pressure dropping funnel under stirring state, use 30ml of acetone rinsed the constant pressure dropping funnel, reacted at 45°C for 12 hours, and then purified the reaction product, with a yield of 95%. FT-IR: ν (cm-1) 3345, 3080, 2956, 2929, 2894, 1715, 1636, 1525, 1453, 1403, 1254, 1167, 777, 652, 602; 1H-NMR (400MHz, CDCl 3 ): δ8.81(s, 1H), 7.87(s, 1H), 6.10(s, 1H), 5.59(s, 1H), 5.31(s, 2H), 5.20(s, 1H), 4.22-4.25( m, 2H), 3.50-3.54 (m, 2H), 1.93 (s, 3H).
Embodiment 2
[0030] (II) The preparation method of monomer is as follows:
[0031] Add 14.3g of 4-methyl-5thiazole ethanol in a 150ml three-necked flask equipped with a magnetic stirring bar, and continuously add 16.28g of methacrylic acid (2-isocyanatoethyl) ester through a constant pressure dropping funnel under stirring , rinse the constant-pressure dropping funnel with 30ml of acetone, react at 20°C for 24 hours, and then purify the reaction product, with a yield of 98%. FT-IR: ν (cm-1) 3362, 3068, 2955, 2929, 2894, 1716, 1635, 1539, 1451, 1414, 1257, 1164, 777, 656; 1H-NMR (400MHz, CDCl 3 ): δ8.60(s, 1H), 6.12(s, 1H), 5.60(s, 1H), 5.07(s, 1H), 4.22-4.26(m, 4H), 3.48-3.52(m, 2H), 3.03-3.11 (m, 2H), 2.41 (s, 3H), 1.95 (s, 3H).
Embodiment 3
[0033] Antibacterial Properties of Dental Resin Containing (I) Monomer
[0034] In this example, the (I) monomer was mixed with the resin system UDMA / TEGDMA system commonly used in dental restorative materials, and the antibacterial properties of the prepared resin after curing were studied. The resin formulation and antibacterial properties are shown in Table 1.
[0035] It can be seen from Table 1 that adding the synthesized (I) monomer to the resin system for dental restoration materials can endow it with effective antibacterial properties.
[0036] Table 1 contains (I) resin composition and cured product antibacterial property
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com