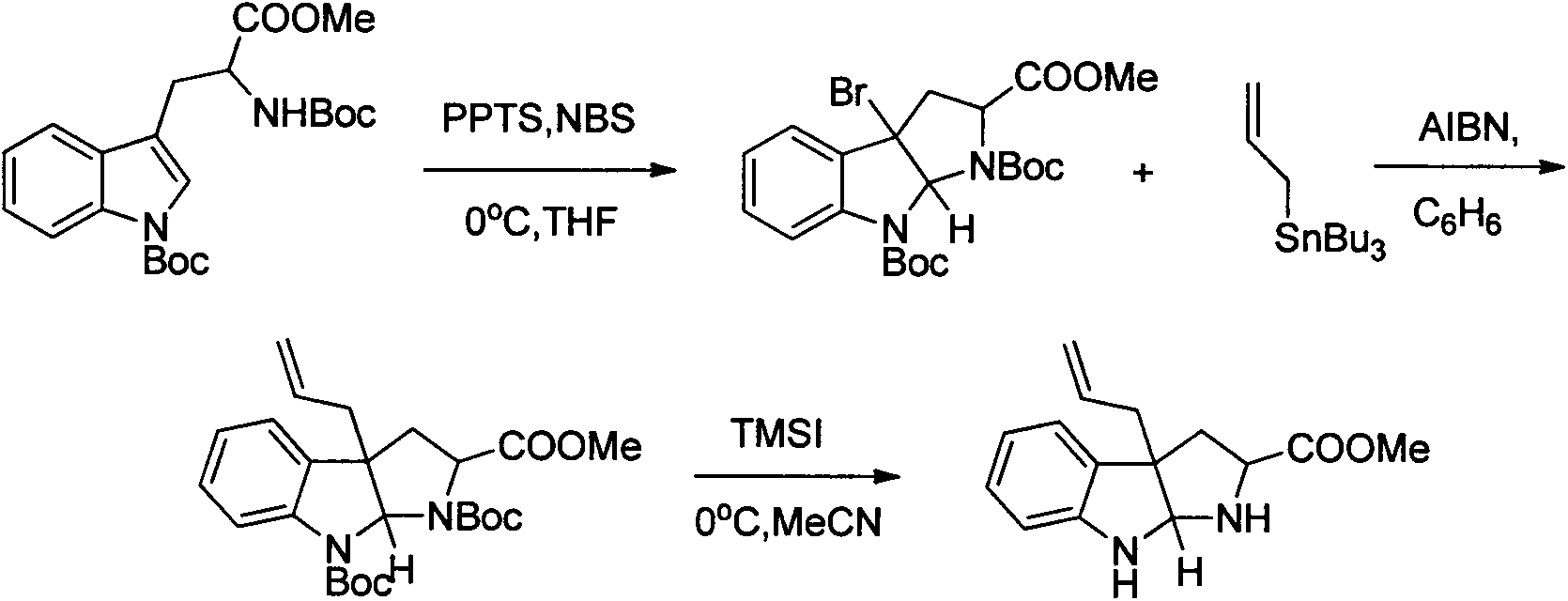
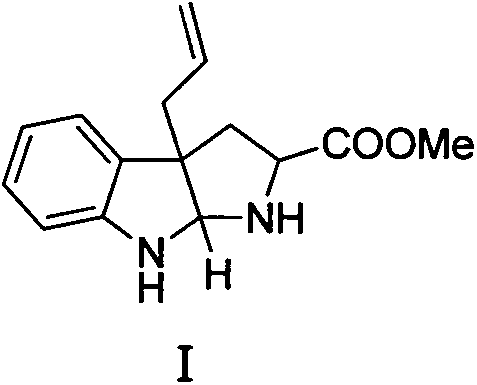
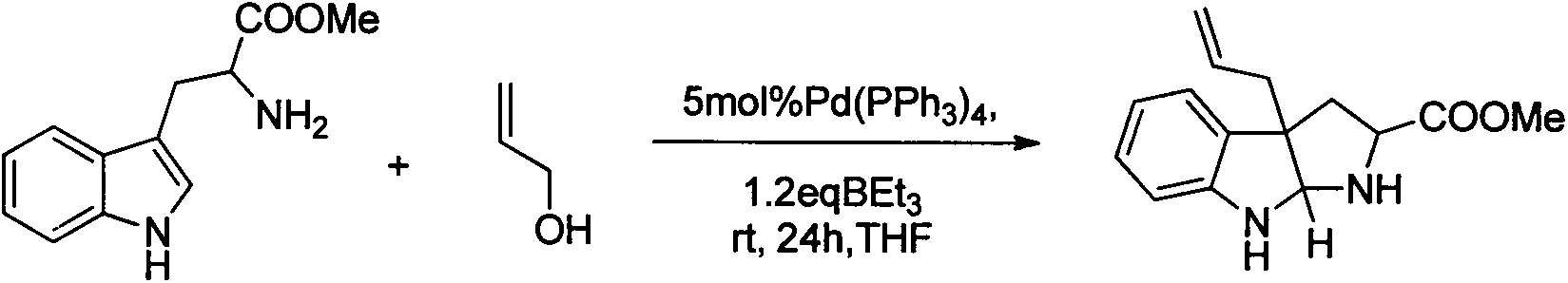Method for preparing natural alkaloid indoline and pyrrolidine compound
A technology for indolinopyrrolidine and alkaloids, which is applied in the field of preparation of natural alkaloid indolinopyrrolidine compounds, and can solve the problems of low total yield, toxicity, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] When R is an acetyl group, the specific operation of the reaction:
[0023] in N 2 Under protection, in a 10ml Schlenk reaction flask, add 217.0mg of tryptophan methyl ester (1.0mmol) and 11.5mg of Pd(dba) successively 2 (5mol%), while stirring, add 2ml of tetrahydrofuran solution, 70ul of allyl acetate (1.0mmol) and 0.7ml of Et 3 1.0 M solution of B in tetrahydrofuran (0.7 mmol). Stirring was then continued at room temperature for 5 h. Afterwards, the reaction solution was diluted with 20.0ml ethyl acetate, and saturated NaHCO 3 After washing with brine, the organic phase was dried with anhydrous sodium sulfate, and 268.2 mg of a colorless liquid (0.89 mmol) was obtained after column separation, with a yield of 89%. The NMR data of Compound I are as follows: 1 H NMR (400MHz, CDCl 3 )δ7.032(t, J=7.0Hz, 2H), 6.724(t, J=7.3Hz, 1H), 6.573(d, J=7.8Hz, 1H), 5.782-5.634(m, 1H), 5.143- 5.022(m, 2H), 4.874(s, 1H), 3.905(dd, J=7.7, 3.4Hz, 1H), 3.352(s, 3H), 3.073-2.794(sb...
example 2
[0025] When R is H, the specific operation of the reaction:
[0026] in N 2 Under protection, in a 10ml Schlenk reaction flask, add 217.0mg of tryptophan methyl ester (1.0mmol) and 11.5mg of Pd(dba) successively 2 (5mol%), while stirring, add 2ml of tetrahydrofuran solution, 68ul of allyl alcohol (1.0mmol) and 0.7ml of Et 3 1.0 M solution of B in tetrahydrofuran (0.7 mmol). Stirring was then continued at room temperature for 8 h. Afterwards, the reaction solution was diluted with 20.0ml ethyl acetate, and saturated NaHCO 3 After washing with brine, the organic phase was dried over anhydrous sodium sulfate, and 247.0 mg of a colorless liquid (0.82 mmol) was obtained after column separation, with a yield of 82%. The NMR data of Compound I are as described above.
example 3
[0028] Use Pd 2 (dba) 3 When being a catalyst, the specific operation of the reaction:
[0029] in N 2 Under protection, in a 10ml Schlenk reaction flask, add 217.0mg of tryptophan methyl ester (1.0mmol) and 45.7mg of Pd successively 2 (dba) 3 (5mol%), 2ml of tetrahydrofuran solution, 70ul of allyl acetate (1.0mmol) and 0.7ml of Et3B in 1.0M tetrahydrofuran solution (0.7mmol) were added while stirring. Stirring was then continued at room temperature for 9 h. Afterwards, the reaction solution was diluted with 20.0ml ethyl acetate, and saturated NaHCO 3 After washing with brine, the organic phase was dried over anhydrous sodium sulfate, and 225.9 mg of a colorless liquid (0.75 mmol) was obtained after column separation, with a yield of 75%. The NMR data of Compound I are as described above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com