Technology for preparing hepatitis B human immunoglobulin for intravenous injection
A technology of human immunoglobulin and hepatitis B, which is applied in the direction of antiviral immunoglobulin and peptide preparation methods, medical preparations of non-active ingredients, etc. Hepatitis surface antibody titer impact and other issues, to achieve the effect of broad market application prospects, high virus safety, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, utilize above-mentioned method to produce intravenous hepatitis B human immunoglobulin:
[0026] (1) Plasma thawing: Thaw raw plasma with hepatitis B surface antibody titer ≥ 8IU / ml at 0-4°C;
[0027] (2) Component separation: Dilute the plasma in step (1) with 0.85% by mass sodium chloride solution to a protein content of 4.5%~5.5%, adjust the pH value to 7.0, add 95% ethanol to The ethanol concentration of the reaction solution is 25%, the temperature of the reaction solution is controlled at -2~-3°C, and pressure filtration is performed to obtain the precipitation of component I;
[0028] (3) Octanoic acid precipitation: Dissolve the component I precipitate in step (2) with 2 times the amount of 0.06M acetate buffer, then adjust the pH to 4.8 with 1mol / L acetic acid, slowly add octanoic acid or caprylate to precipitate, Filtrate is collected by filtration;
[0029] (4) The first step of chromatography: adjust the filtrate in step (3) to pH 6.2 with ph...
Embodiment 2
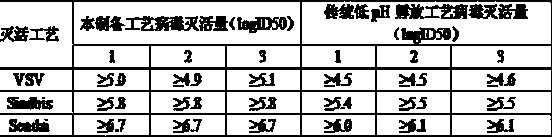
[0034] Embodiment 2, this preparation method inactivated virus technique compares with low pH hatching put inactivated virus technique:
[0035]
[0036] Therefore, the virus inactivation method used in this process is more effective than the traditional low pH virus inactivation method.
Embodiment 3
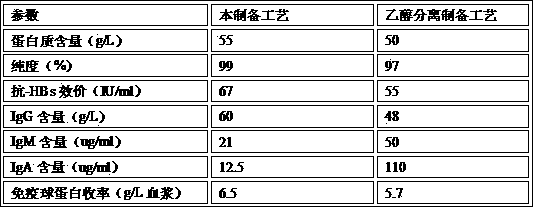
[0037] Embodiment 3, preparation technique of the present invention and traditional ethanol separation preparation technique gained product parameter comparison:
[0038]
[0039] Therefore, the quality and yield of the intravenously injected hepatitis B human immunoglobulin prepared by this process are better than those prepared by traditional ethanol separation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com