Extremely acidic pH fluorescent probe, and preparation method and application thereof
A fluorescent probe, acid technology, applied in the field of fluorescent probes, to achieve the effects of reducing interference, great practical value, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of an extremely acidic pH fluorescent probe based on benzindole:
[0025]
[0026] (1) Under the protection of inert gas, mix 0.283g (1.35mmol) 1,1,2-trimethyl-1H-benzo[e]indole with 0.454g (1.48mmol) 3-[3-(4-Fluorophenyl)-1-isopropyl-1H-indol-2-yl]-propenal Dissolve in 6 mL of DMF, add 0.378 g (6.75 mmol) of KOH solid, stir at room temperature, and react overnight.
[0027] (2) KOH solid was removed by filtration, H2O (6 mL) was added to the reaction solution, and the mixed solution was mixed with CH 2 Cl 2 Extraction (10ml x 3). The combined organic phases were washed with anhydrous Na 2 SO 4 After drying, the crude product was obtained by distillation under reduced pressure.
[0028] (3) The crude product was separated on a silica gel column with dichloromethane as the eluent to obtain an orange-yellow solid. 1 H NMR (300MHz, DMSO-d 6 ), δ: 1.556(s, 6H), 1.648-1.671(d, 6H), 5.009-5.055(m, 1H), 6.607-6.658(m, 2H), 7.037-7.085(m, 1H), 7.178-7.258...
Embodiment 2
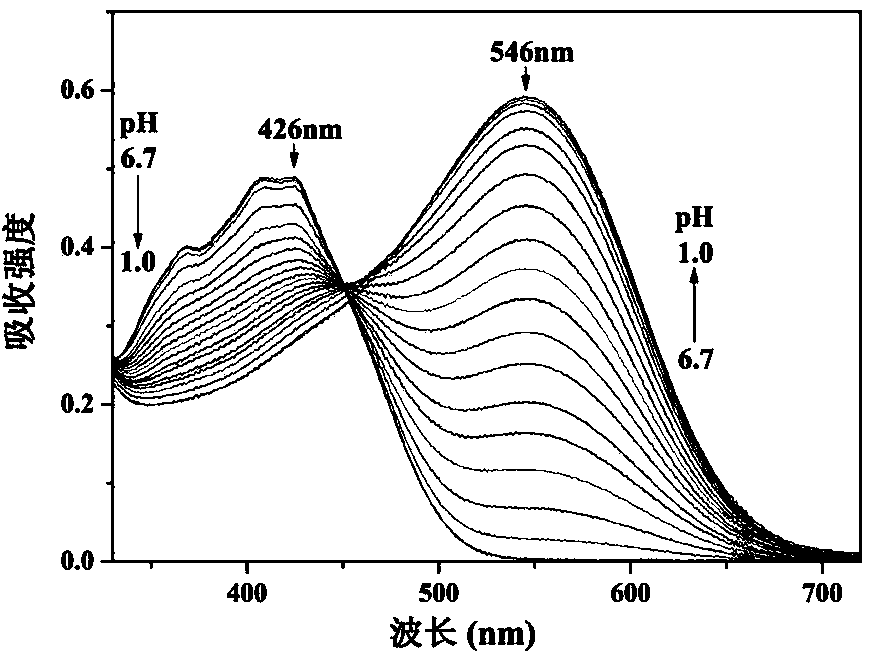
[0030] The probe concentration in Example 1 was maintained at 5 μmol / L, the pH was adjusted with 1 mol / L HCl in the ethanol / water (volume ratio of 2:1) system, and its absorption spectrum was recorded ( figure 1 ). With the decrease of pH value, the absorption peak at the short wavelength of 426 nm gradually decreased, the absorption peak at the long wavelength of 546 nm was significantly enhanced, and there was an isoabsorption point at 450 nm. The color of the solution also changed from bright yellow to red ( figure 2 ).
Embodiment 3
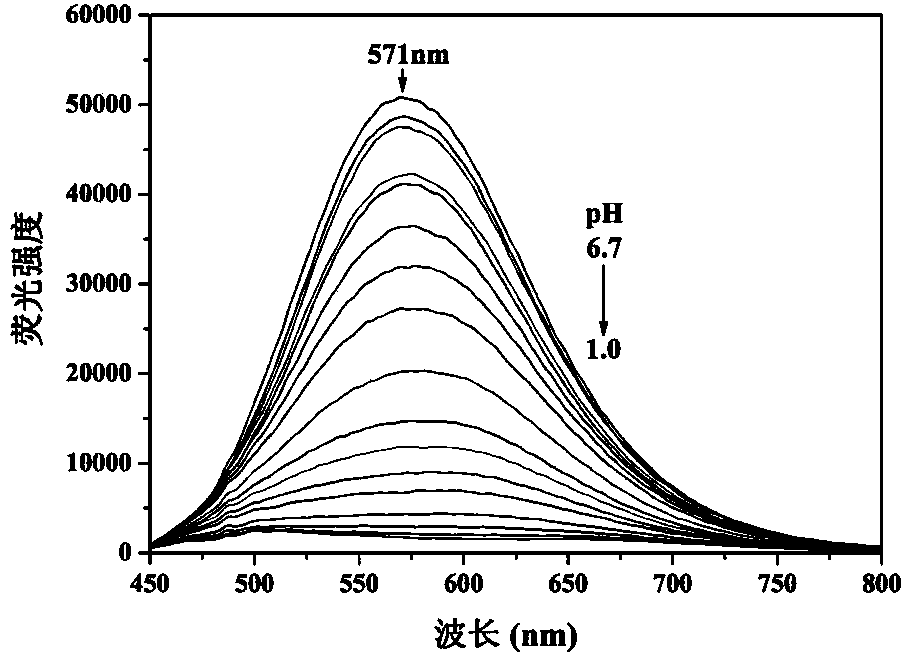
[0032] The probe concentration in Example 1 was kept at 5 μmol / L, the pH value was adjusted with 1 mol / L HCl in the ethanol / water (volume ratio of 2:1) system, and the fluorescence emission spectrum was recorded with 426 nm as the excitation wavelength ( image 3 ). The fluorescence peak at 571 nm gradually weakened with the decrease of pH value. Fluorescence intensity at 571 nm was plotted against pH and fitted with sigmoidal ( Figure 4 ), the pKa value of the probe in Example 1 was obtained as 2.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com