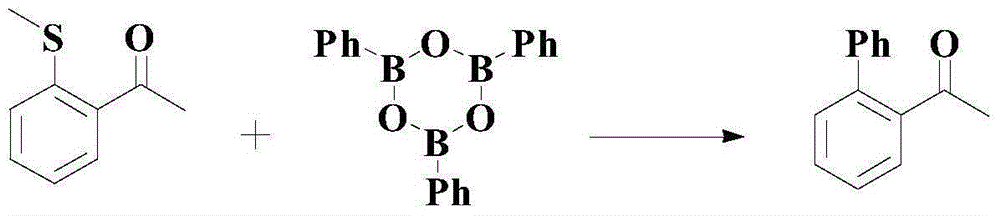
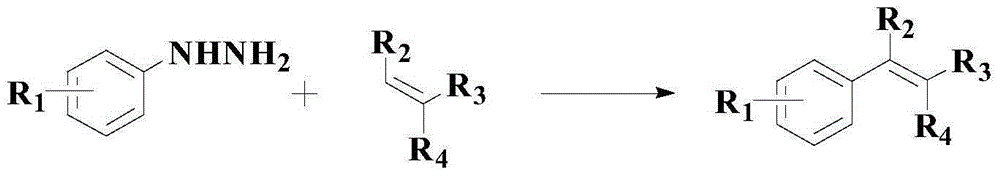
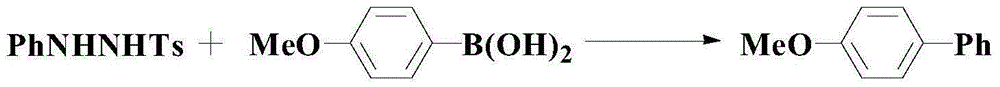A kind of catalytic synthesis method of pharmaceutical intermediate diaryl compounds
A synthesis method and compound technology are applied to the catalytic coupling of aryl hydrazine and aryl boronic acid to prepare diaryl compounds, and the field of catalytic synthesis of pharmaceutical intermediate diaryl compounds, which can solve the problem of low reaction yield and suitable substrates. narrow range, harsh reaction conditions and other problems, to overcome the effect of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] In the reactor, add 1mmol formula (I) compound and 2.5mmol formula (II) compound successively, then add 0.06mmolPd(acac) 2 , 0.35mmol triphenylphosphine and 1.5mmol acetic acid, then add 4.2ml solvent toluene, add 55mg mass ratio of 0.3:1 porphyrin and 1-benzyl-3-methylimidazolium bromide additive mixture under stirring , warming up to 70°C and insulated for 2.5 hours, TLC monitors the end point of the reaction, after the reaction is completed, the mixture is concentrated in vacuo, and the residue is purified by silica gel column chromatography to obtain the compound of formula (III), with a yield of 97.5% and a purity of 99.3 % (HPLC).
[0036] 1 HNMR (400MHz, CDCl 3 ) δ 7.65-7.62 (m, 2H), 7.52-7.47 (m, 4H), 7.43-7.38 (m, 2H), 7.25 (d, J=7.40Hz, 1H), 2.46 (s, 3H).
Embodiment 2
[0038]
[0039] In the reactor, add 1mmol formula (I) compound and 3mmol formula (II) compound successively, then add 0.07mmolPd(acac) 2, 0.4mmol triphenylphosphine and 2mmol acetic acid, then add 4.6ml solvent toluene, add 52mg mass ratio under stirring and be the auxiliary agent mixture of the porphyrin of 0.3:1 and 1-benzyl-3-methylimidazolium bromide, The temperature was raised to 75° C. and kept for reaction for 2 hours. The end point of the reaction was monitored by TLC. After the reaction was completed, the mixture was concentrated in vacuo, and the residue was purified by silica gel column chromatography to obtain the compound of formula (III), with a yield of 98.3% and a purity of 99.1% ( HPLC).
[0040] 1 HNMR (400MHz, CDCl 3 )δ7.62-7.58 (m, 4H), 7.52-7.48 (m, 2H), 7.43-7.38 (m, 1H), 7.17-7.12 (m, 2H).
Embodiment 3
[0042]
[0043] In the reactor, add 1mmol formula (I) compound and 2mmol formula (II) compound successively, then add 0.04mmolPd(acac) 2 , 0.3mmol triphenylphosphine and 1.8mmol acetic acid, then add 5ml solvent toluene, add 60mg mass ratio under stirring and be the auxiliary agent mixture of the porphyrin of 0.3:1 and 1-benzyl-3-methylimidazolium bromide, Raise the temperature to 80°C and keep it warm for 1.5h. The end point of the reaction was monitored by TLC. After the reaction was completed, the mixture was concentrated in vacuo, and the residue was purified by silica gel column chromatography to obtain the compound of formula (III), with a yield of 98.5% and a purity of 99.0%. (HPLC).
[0044] 1 HNMR (400MHz, CDCl 3 )δ8.43(t,J=1.96Hz,1H),8.22-8.18(m,1H),7.95-7.93(m,1H),7.65-7.61(m,3H),7.53-7.49(m,2H) ,7.44-7.41(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com