Use of 2-(substituted phenylamino) benzoic acid and ester compound thereof in preparation of FTO (Fat Mass and Obesity-Associated Protein) inhibitor
A technology of compound and application, applied in the field of preparation of selective FTO inhibitors, capable of solving problems such as lack of FTO inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment Embodiment 1
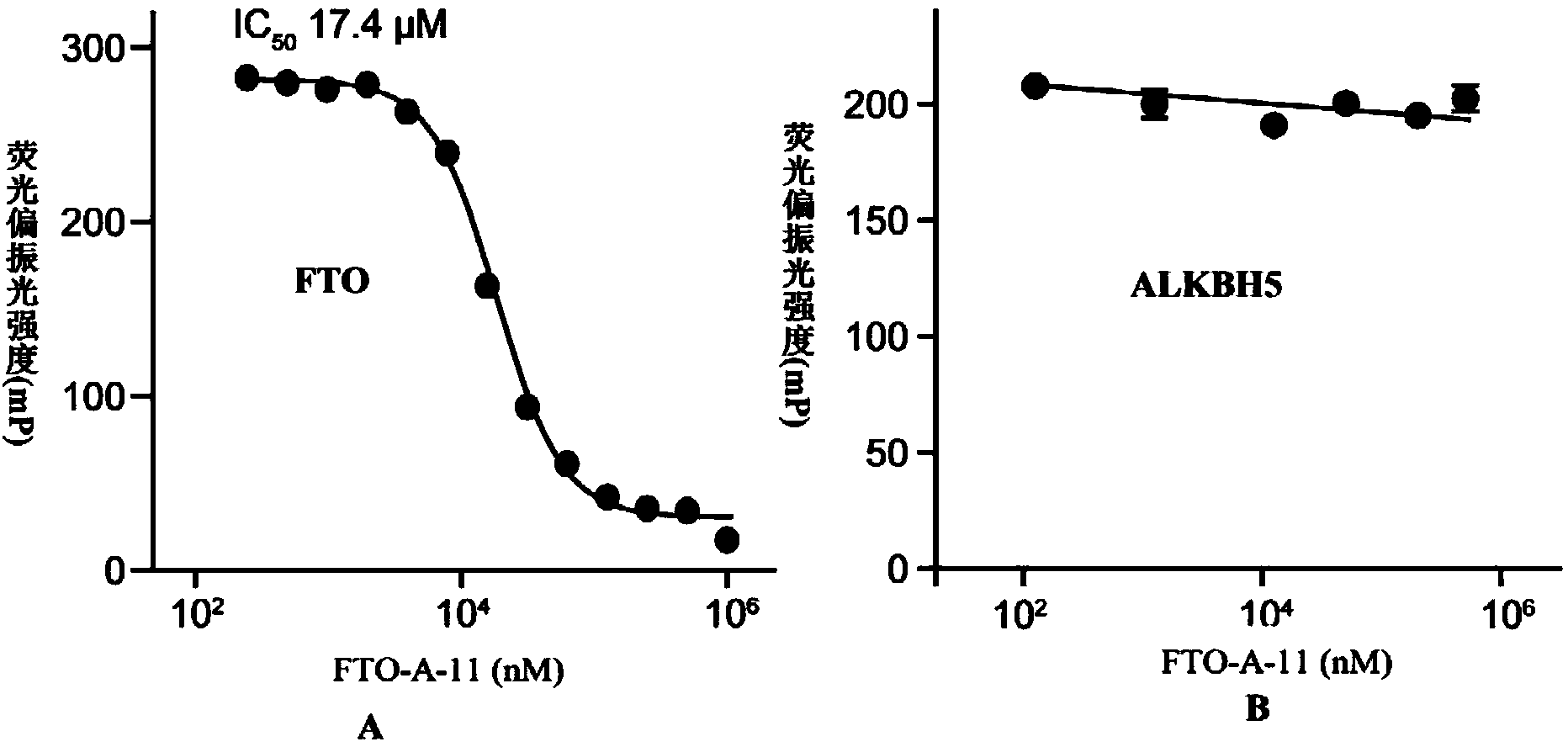
[0131] Experimental example 1: Fluorescence polarization test detects the selective inhibition of FTO and ALKBH5 by 2-(substituted anilino)benzoic acid and its esters
[0132] The substrate for the fluorescence polarization experiment is fluorescently labeled ssDNA with the sequence 5'-ATTGTCA(m 6 A) CAGCAGA-FAM-3'. The reaction solution contains 50mM boric acid buffer (pH7.5), 20nM ssDNA, 3μM FTO or 3μM ALKBH5, and the final volume is 100μL. After incubation at room temperature for 30min, the fluorescence polarization value is read by a microplate reader, and the detection wavelength is the excitation light at 480nm and the emission Light 520nm. Inhibition curves and parameters via GraphPad Prism5.0 TM The nonlinear fitting is carried out. The fluorescence polarization value represents the binding force parameter between the protein and the substrate ssDNA. From the attached figure 1 In Figure A, it can be seen that compound FTO-A-11 can reduce the polarization value in a ...
experiment Embodiment 2
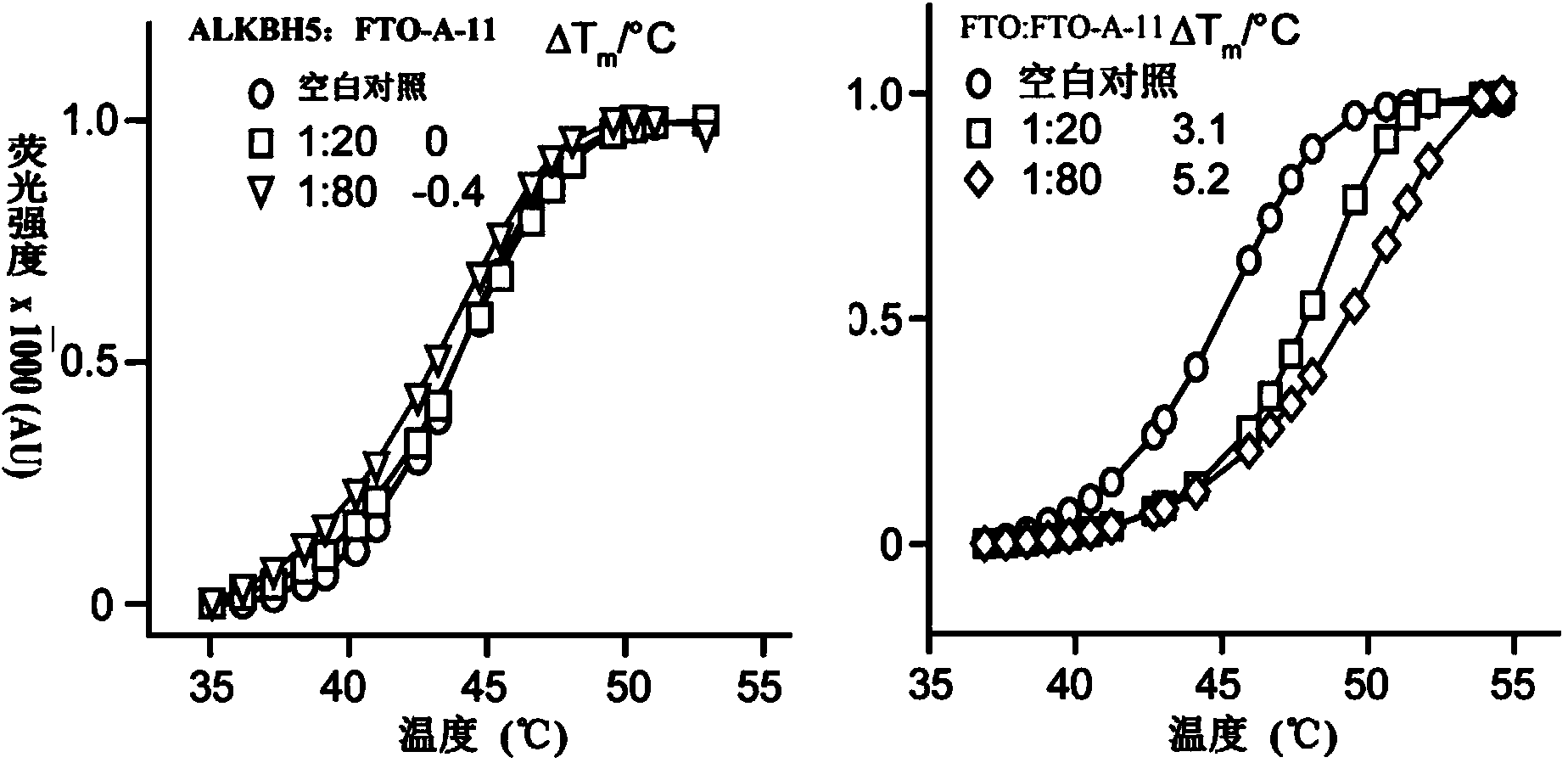
[0133] Experimental Example 2: Differential Scanning Fluorescence Experiment Detects the Selective Inhibition of 2-(Substituted Anilino)benzoic Acid and Its Esters to FTO and ALKBH5
[0134] The reaction system was 5×SYPRO Orange dye, FTO-A-11 concentration gradient compound, 2 μM FTO or ALKBH5, and 1.5% DMSO was used as a control. The experiment was tested by an ABI7500 quantitative PCR instrument, and the temperature of the reaction solution rose from 25°C to 95°C with a rate of 1% slope. The excitation wavelength and emission wavelength were 492nm and 610nm, respectively. All experiments were repeated three times. Melting curve by Graphpad Prism5.0 TM to fit. From attached figure 2 In Figure B, it can be seen that compound FTO-A-11 has inhibitory activity on FTO, and it can be seen from Figure A that compound FTO-A-11 has no inhibitory activity on ALKBH5, indicating that FTO-A-11 can selectively inhibit FTO activity. However, it has no effect on ALKBH5 activity.
experiment Embodiment 3
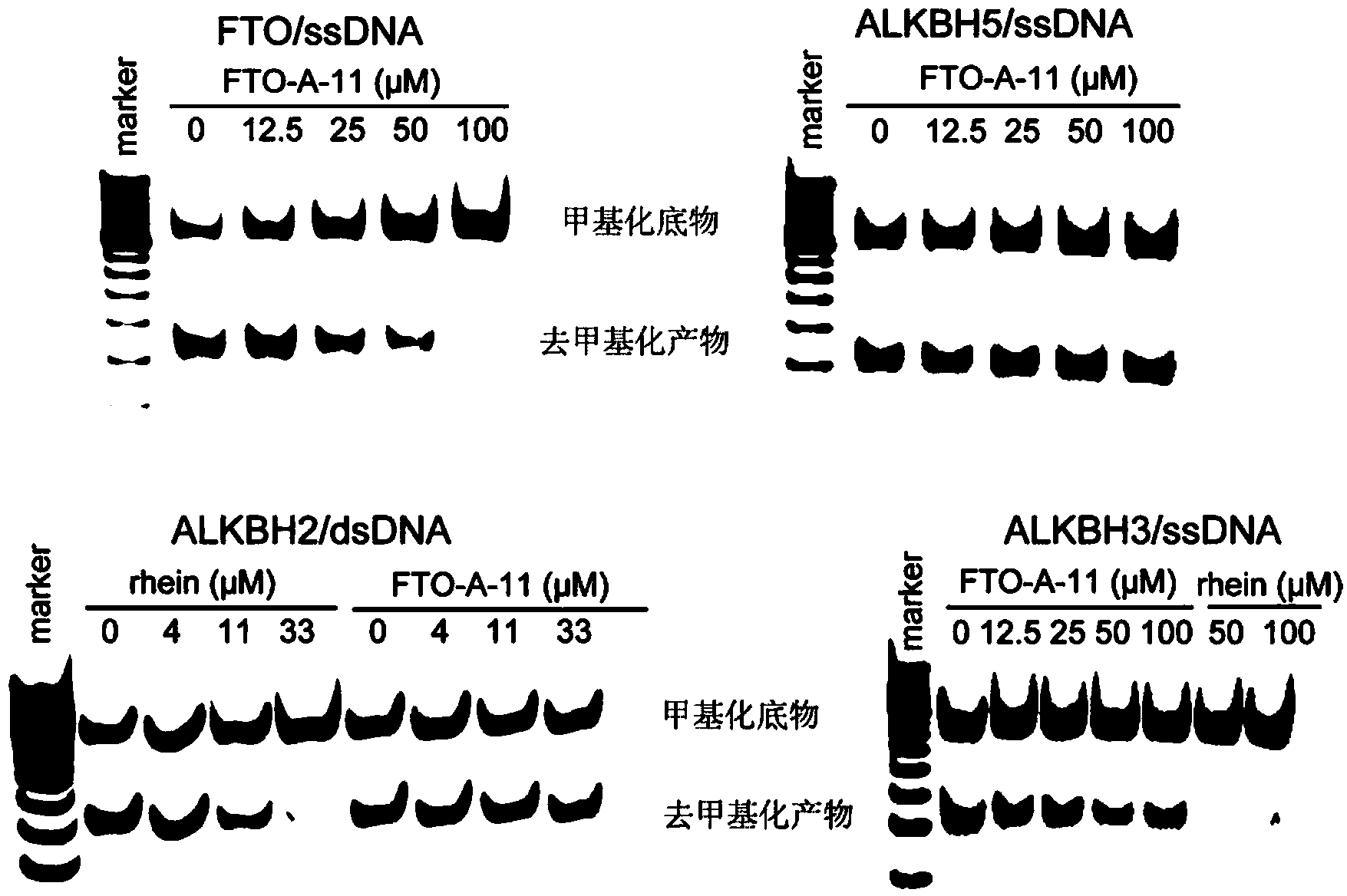
[0135] Experimental Example 3: Non-denaturing polyacrylamide gel electrophoresis test for selective inhibition of FTO and ALKBH5 by 2-(substituted anilino)benzoic acid and its esters
[0136] The reaction substrate single-stranded DNA (ssDNA) sequence is 5'-TAGACATTGCCATTCTCGATAGG(m 6 A) TCCGGTCAAACCTAGACGAATTCCA-3', which contains a DpnII restriction site. The reaction system was 50 mM tris(hydroxymethyl)aminomethane-hydrochloric acid buffer (pH7.5), 1 μM ssDNA, 300 μM α-ketoglutarate (2OG), 280 μM ferrous ammonium sulfate ((NH 4 ) 2 Fe(SO 4 ) 2 ), 2 mM L-ascorbic acid, concentration gradient compound FTO-A-11, 1 μM FTO, ALKBH2, ALKBH3 or ALKBH5. After the reaction solution was incubated at room temperature for 2 h, it was quenched by heating. The ssDNA was annealed with its complementary strand to form a double-stranded DNA, which was digested with DpnII, then detected by 15% non-denaturing polyacrylamide gel electrophoresis, and the intensity of the band was detected a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com