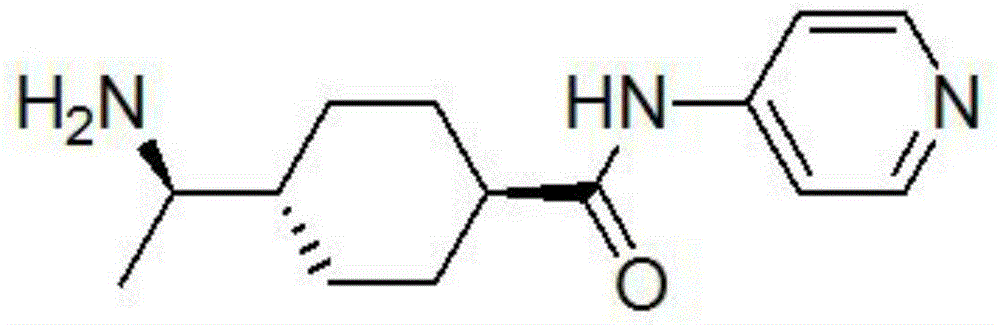
A kind of preparation method of rho kinase inhibitor y27632 compound
A technology of Y27632 and kinase inhibitors, which is applied in the field of compound preparation, can solve the problems of expensive rhodium catalysts and impracticality, and achieve the effects of good reactions in each step, suitable for large-scale production, and easy acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
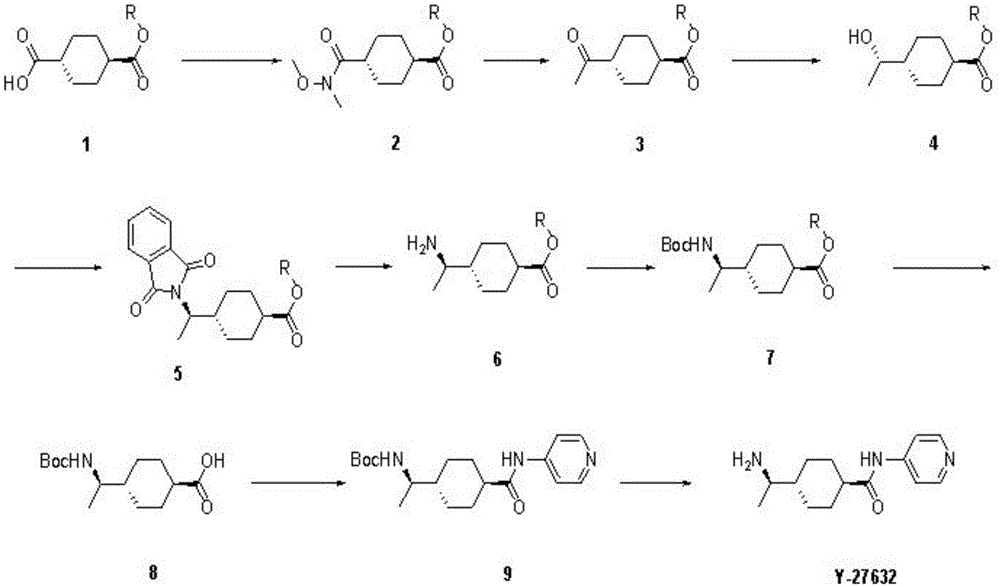
[0024] like figure 1 As shown, the present invention discloses a preparation method of a Rho kinase inhibitor Y27632 compound, comprising the following steps:
[0025] a, raw material compound 1 namely (trans)-1,4-cyclohexanedicarboxylic acid monoester and dimethyl hydroxylamine are added to N 2 In a protected three-necked flask, dichloromethane and EDCI were subsequently added. Add N,N'-dimethylaminopyridine at 0°C and stir overnight at room temperature. The reaction solution was washed with water and extracted with dichloromethane, the organic phases were combined, and then successively washed with hydrochloric acid, saturated NaHCO 3 , water and saturated NaCl solution. The organic phase was dried and concentrated by filtration to obtain compound 2 (trans)-4-(N-methoxy-N-methylformamide)cyclohexylcarboxylate.
[0026] b. Add the compound 2 into a three-necked flask, and then add THF. in N 2 Under protection, the temperature was lowered to -20°C, kept for 10 minutes, a...
Embodiment 1
[0036] A preparation method of a Rho kinase inhibitor Y27632 compound, the method comprising the following steps:
[0037] a. Add raw material compound 1, trans-monomethyl cyclohexanedicarboxylate (100g, 537.03mmol) and dimethylhydroxylamine (63.05g, 644.43mmol) into a 2L three-necked flask protected by nitrogen, and then add dichloromethane (1500 mL) and EDCI (154.42 g, 805.54 mmol). The reaction flask was cooled to 0°C in an ice-water bath, and then N,N'-dimethylaminopyridine (65.52g, 537.03mmol) was added, the reaction system changed from colorless to yellow, and the temperature of the reaction system was raised to Stir overnight at room temperature. The reaction solution was mixed with 500mLH 2 O water was washed three times, and the aqueous phase was extracted twice with 200 mL of dichloromethane. The organic phases were combined, and then successively washed with 600mL of 0.5N hydrochloric acid, saturated NaHCO 3 , water and saturated NaCl solution twice each. The o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com