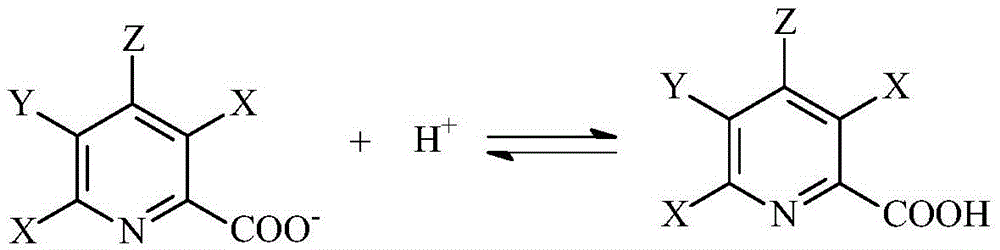
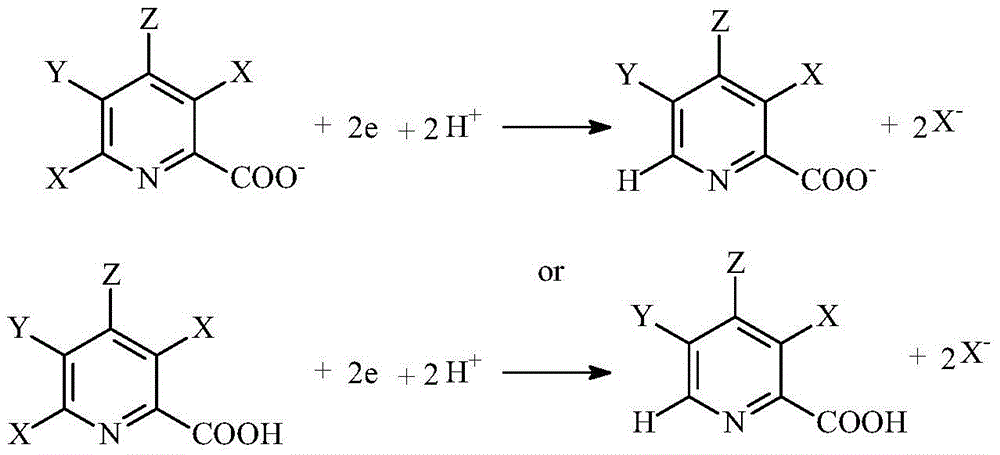
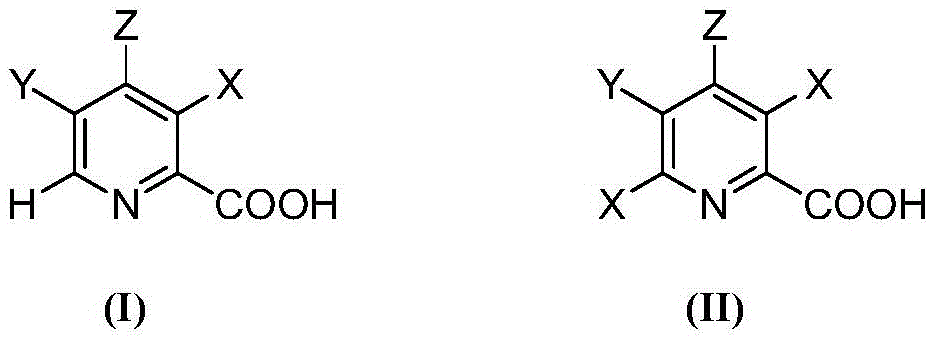Selective electrochemical reduction method of halogenated picolinic acid or its salt compounds
A technology of halogenated picolinic acid and salt compounds, which is applied in the field of halogen substituents at 6-position, can solve the problem of not proving 3- and 6-halogens, etc., and achieve the effect of reducing and removing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 active silver electrode
[0041] In the diaphragmless electrolyzer, an expanded screen silver was used as the working electrode with an apparent size of 0.1 cm × 10 cm × 10 cm, graphite with the same apparent size was used as the anode, and the anode and cathode were separated by 2 cm. The electrolytic solution is 1000mL of 2.9wt% sodium chloride+2wt% sodium hydroxide aqueous solution, and the electrolytic solution remains static. Then the silver electrode is oxidized by direct current, the current density is 50mA / cm 2 , after the electrode potential drops to 0.9 volts, the polarity is reversed to reduce the silver electrode, and the current density is 100mA / cm 2 After the electrode potential rises to -0.8 volts, the power supply is stopped, the reaction temperature is controlled at 25°C, and the cell voltage is 2.5-3.5V. Take out the silver electrode and immerse it in deionized water for later use.
Embodiment 2
[0042] Embodiment 2 Electrolytic synthesis of 3,5-dichloropicolinic acid (3,5-D)
[0043] The diaphragm plate frame tank is the electrolytic reactor, the perfluorosulfonic acid membrane is the diaphragm, the activated expanded screen silver is the cathode, and the mesh 316 stainless steel is the anode. The 0.25mol / L phosphate buffer solution of 1000mLpH=4 is catholyte, and the quality of throwing 3,5,6-triclopyridine carboxylic acid (3,5,6-T, 98wt%) in catholyte for the first time is 20 grams; 4wt% Sodium hydroxide aqueous solution is the anolyte. During the electrolysis process, use 30wt% sulfuric acid to control the pH of the catholyte to 3-5, add 30 grams of 3,5,6-T to the catholyte step by step, control the temperature to 30-35°C, and control the current density to 3A / dm 2 . Stop electrolysis after feeding 4F / mol3,5,6-T electricity. After transferring the catholyte to the beaker, add sulfuric acid to adjust the pH=1, then control the temperature of the catholyte to 5°...
Embodiment 3~ Embodiment 9
[0045] Embodiment 3 to Embodiment 9 were carried out according to the experimental parameters in Table 1, and the rest of the operations were the same as in Embodiment 2.
[0046] Table 11000mL scale electrolytic dechlorination to prepare 3,5-D experimental conditions and results a
[0047]
[0048]
[0049] a The activated expanded screen silver is used as the cathode, and the electricity of 4F / mol reaction substrate is passed through.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com