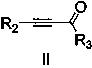
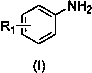A kind of preparation method of quinoline derivative
A technology of derivatives and quinoline, applied in the field of quinoline derivatives, can solve the problems of difficult by-products, high reaction temperature, harsh reaction conditions, etc., and achieve the effect of easy separation and purification, simple operation and single product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of 2,4-diphenylquinoline: Add 0.5mmol (46.5mg) of aniline, 0.005mmol (1.29mg) of catalyst AgOTf, 0.5mmol (103mg) of 1,3-diphenylpropynone into the reaction vessel, Toluene 2mL. at 60 o React in a C oil bath for 10 hours, cool to room temperature, extract the product with ethyl acetate, concentrate under reduced pressure, and purify the product by column chromatography to obtain a white solid product with a yield of 98%. 1 HNMR (500MHz, CDCl 3 ) ppm: 8.43 (d, J =8.0Hz,1H),8.34(d, J =8.0Hz,2H),8.00(d, J =8.5Hz,1H),7.92(s,1H),7.80(t,1H),7.51-7.64(m,9H); 13 CNMR (500MHz, CDCl 3 ):156.90,149.23,149.05,139.77,138.56,130.35,129.70,129.60,129.50,128.96,128.72,128.52,127.75,126.47,125.92,125.75,119.39); 21 h 15 N:[M + ], 281.1207; Found: 281.1204.
Embodiment 2
[0027] Preparation of 4-phenylquinoline: Add 0.5mmol (46.5mg) of aniline, 0.005mmol (1.29mg) of catalyst AgOTf, 0.5mmol (65mg) of 3-phenylpropynaldehyde, and 2mL of toluene into a reaction vessel. at 60 o React in a C oil bath for 10 hours, cool to room temperature, extract the product with ethyl acetate, concentrate under reduced pressure, and purify the product by column chromatography to obtain a white solid product with a yield of 94%.
Embodiment 3
[0029] Preparation of 4-phenyl-2-p-tolylquinoline: Add 0.5mmol (46.5mg) of aniline, 0.005mmol (1.29mg) of catalyst AgOTf, 3-phenyl-1-p-tolyl-?? Propyrone 0.5mmol (11.0mg), toluene 2mL. at 60 o React in a C oil bath for 8 hours, cool to room temperature, extract the product with ethyl acetate, concentrate under reduced pressure, and purify the product by column chromatography to obtain a white solid product with a yield of 92%. 1 HNMR (500MHz, CDCl 3 ) ppm: 8.36 (d, J =8.0Hz,1H),8.25(d, J =8.0Hz,2H),7.95-7.93(d, J =8.0Hz,1H),7.85(s,1H),7.81-7.74(m,1H),7.61-7.52(m,6H),7.38-7.36(d, J =8.0Hz,2H),2.46(s,3H); 13 CNMR (500MHz, CDCl 3 ):156.9,149.2,148.6,139.4,138.4,136.7,120.2,129.6,129.5,128.5,128.2,127.4,126.1,125.6,125.5,119.1,21.5;HRMS(EI)Calcd.forC 22 h 17 N:[M + ], 295.1369; Found: 295.1365.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com