A cheap and large-scale method for preparing nitrogen-based metal compound hydrogen storage materials
A hydrogen storage material and base metal technology, applied in the production of hydrogen and other directions, can solve the problems of difficulty in practicalization and popularization, and achieve the effects of cost reduction, simple process, and strong safety and operability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
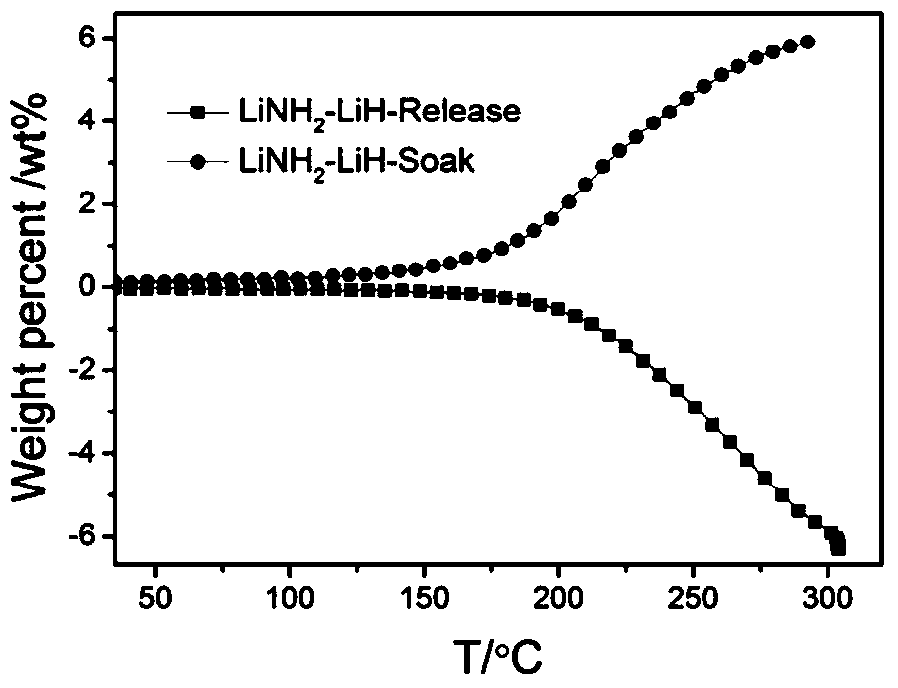
[0015] Embodiment 1: prepare Li-N-H (LiNH 2 -LiH) system
[0016] (1) Weigh a certain amount of lithium metal, such as 70g, in a glove box, then add it to a ball mill jar, and then inject 10-120 atm of ammonia gas or liquid ammonia.
[0017] (2) After adding metal lithium and ammonia gas as described in step (1), place the ball mill jar on a planetary ball mill at 100-200 rpm and mix for 1-20 hours, and at the same time make part of the metal lithium and ammonia gas react to form lithium amide.
[0018] (3) Take out the sample mixed in step (2) and place it in a pressure-resistant reactor for ammoniation, the ammoniation temperature is between 50 and 350°C; the time is 1-50h; the steps can also be repeated for a long time (>50h) (2) Until all metallic lithium is transformed into lithium amide.
[0019] (4) Perform a temperature-programmed (fixed heating rate) deamination test (TPD-MS) on the lithium amide sample obtained after ammoniation in step (3) to determine the deamina...
Embodiment 2
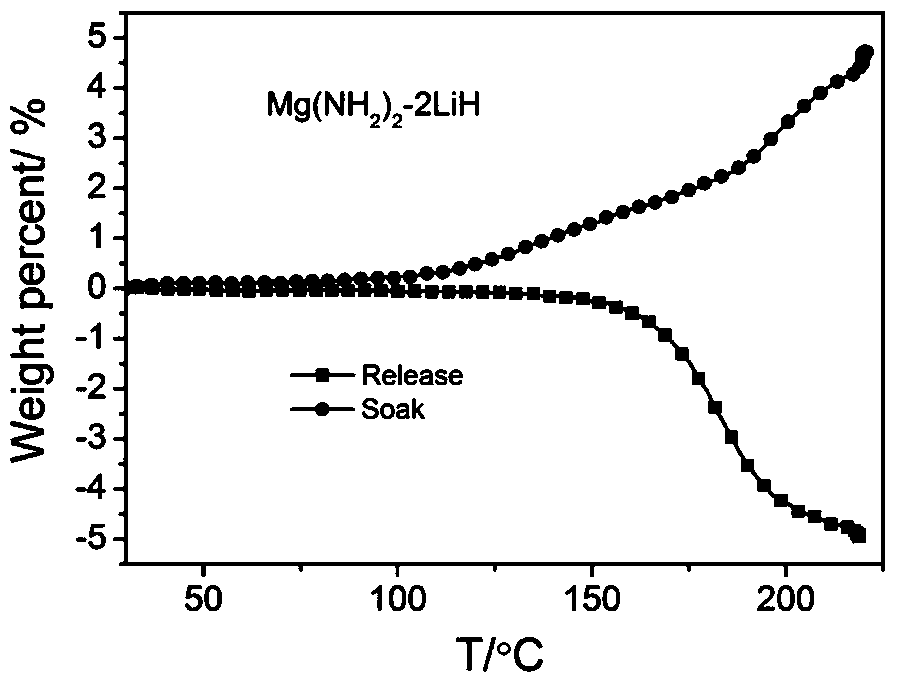
[0022] Embodiment 2: prepare Li-Mg-N-H (Mg (NH 2 ) 2 -nLiH) system
[0023] (1) Take a certain amount of metallic magnesium and a corresponding amount of metallic lithium (such as Mg(NH 2 ) 2 -2LiH system, the molar ratio of Mg and Li is 1:2, such as a total of 38g), and then added to the ball mill jar, followed by injection of ammonia gas (>5atm) or liquid ammonia.
[0024] (2) After adding metal magnesium, metal lithium and ammonia gas as described in step (1), place the ball mill jar on a planetary ball mill at 50-200rpm and mix for more than 1 hour until uniform, and at the same time make part of the metal lithium or magnesium react with ammonia gas Generate lithium amide or magnesium amide.
[0025] (3) Take out the sample mixed in step (2) and place it in a pressure-resistant reactor for ammoniation, and the ammoniation temperature is between 150 and 400°C;
[0026] (4) The temperature-programmed deamination test (TPD-MS) was performed on the samples obtained after ...
Embodiment 3
[0029] Embodiment 3: prepare KH modified Li-Mg-N-H (Mg(NH2 ) 2 -nLiH-yKH) system
[0030] (1) Take a certain amount of metal magnesium and a corresponding amount of metal lithium and metal potassium (such as Mg(NH 2 ) 2 -1.9LiH-0.1KH system, the molar ratio of Mg, Li and K is 1:1.9:0.1, such as a total weight of 41.2g), then add it to the ball mill tank, and then inject ammonia gas (>2atm) or liquid ammonia.
[0031] (2) After adding metal magnesium, metal lithium, metal potassium and ammonia gas as described in step (1), place the ball mill jar on a planetary ball mill and mix at 50-200rpm for > 1h until the mixture is uniform, and at the same time make part of the metal lithium, Metal potassium or magnesium reacts with ammonia gas to generate lithium amide, potassium amide or magnesium amide.
[0032] (3) Take out the sample mixed in step (2) and place it in a pressure-resistant reactor for ammoniation, and the ammoniation temperature is between 150 and 400°C;
[0033] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com