Method for efficiently synthesizing quinoline
A derivative, quinoline technology, applied in the field of quinoline derivatives, can solve the problems of limited quinoline derivatives, high reaction temperature, harsh reaction conditions, etc., and achieve the effects of easy separation and purification, single product, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
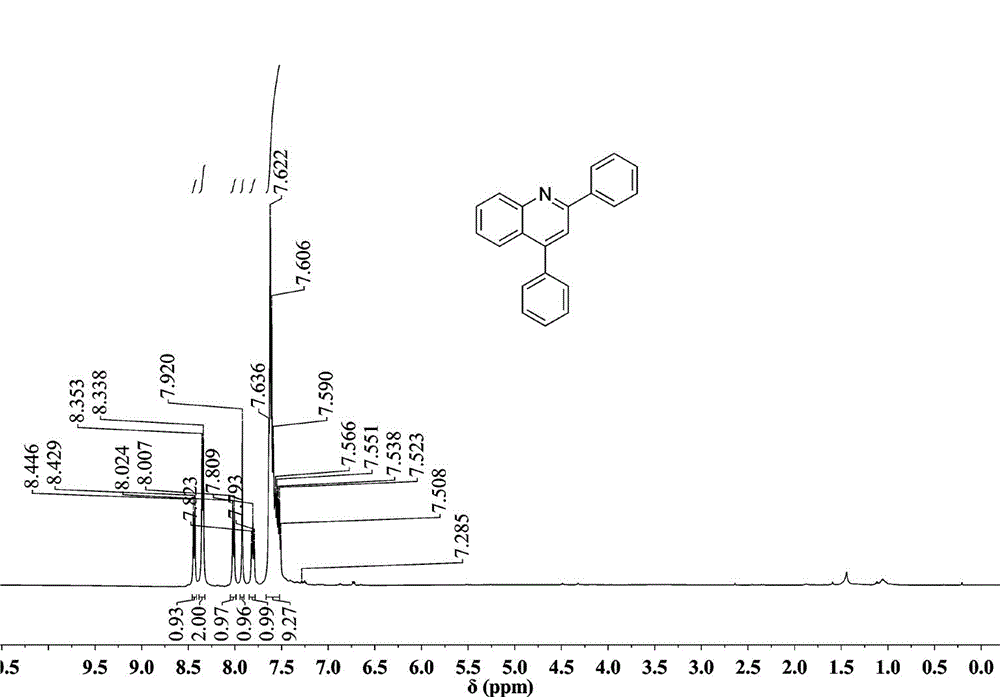
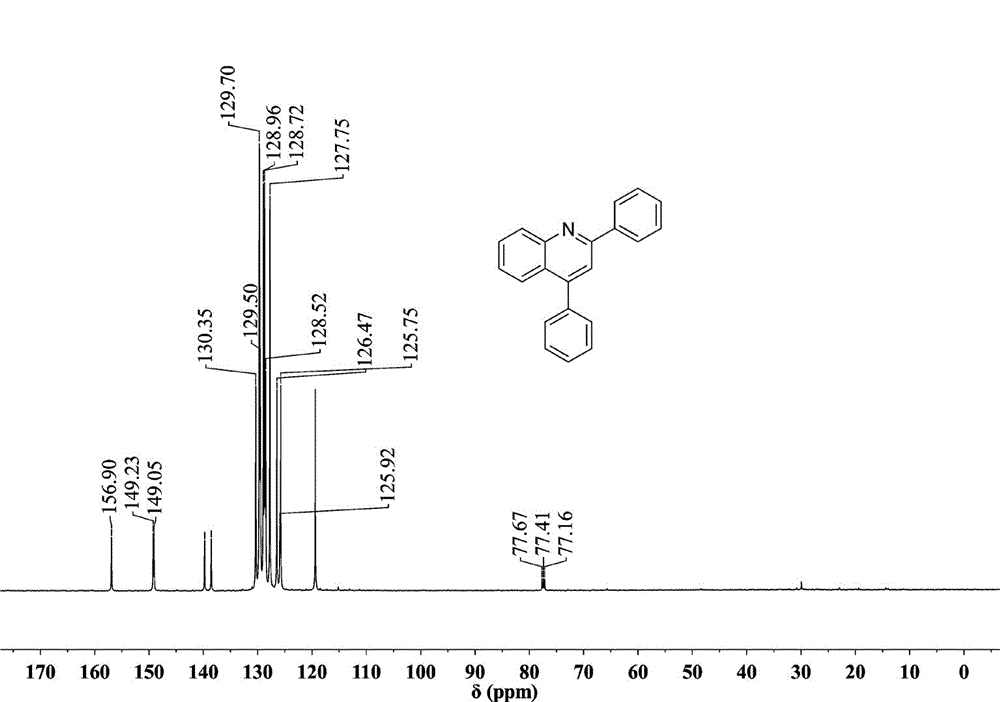
[0027] Preparation of 2,4-diphenylquinoline: Add 0.5 mmol (46.5 mg) of aniline, 0.005 mmol (1.29 mg) of catalyst AgOTf, 0.5 mmol (104 mg) of 1,3-diphenylpropenone into the reaction vessel, Toluene 2mL. at 110 o React in a C oil bath for 10 hours, cool to room temperature, extract the product with ethyl acetate, concentrate under reduced pressure, and purify the product by column chromatography to obtain a white solid product with a yield of 96%. 1 H NMR (500 MHz, CDCl 3 ) ppm: 8.43 (d, J = 8.0 Hz, 1H), 8.34 (d, J = 8.0 Hz, 2H), 8.00 (d, J = 8.5 Hz, 1H), 7.92 (s, 1H), 7.80 (t, 1H), 7.51 - 7.64 (m, 9H); 13 C NMR (500 MHz, CDCl 3 ): 156.90, 149.23, 149.05, 139.77, 138.56, 130.35, 129.70, 129.60, 129.50, 128.96, 128.52, 127.75, 125.92, 125.75, 119.39; 21 h 15 N: [M+ ], 281.1207; Found: 281.1204.
Embodiment 2
[0029] Preparation of 4-phenylquinoline: Add 0.5 mmol (46.5 mg) of aniline, 0.005 mmol (1.29 mg) of catalyst AgOTf, 0.5 mmol (66 mg) of 3-phenylacrolein, and 2 mL of toluene into a reaction vessel. at 110 o React in a C oil bath for 10 hours, cool to room temperature, extract the product with ethyl acetate, concentrate under reduced pressure, and purify the product by column chromatography to obtain a white solid product with a yield of 90%.
Embodiment 3
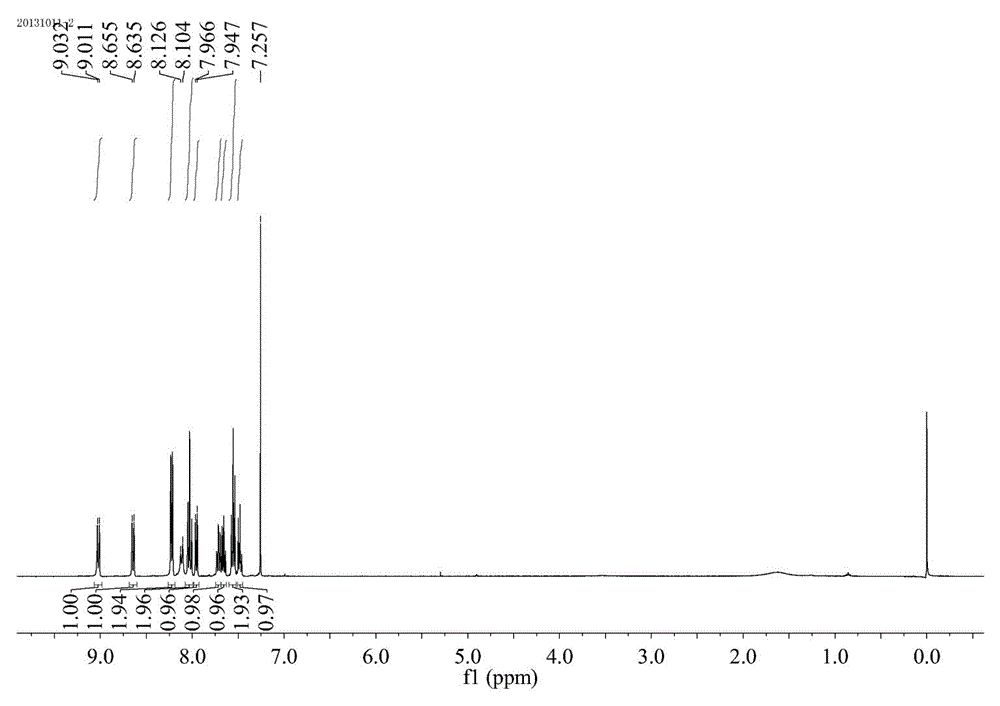
[0031] Preparation of 4-phenyl-2-p-tolylquinoline: 0.5 mmol (46.5 mg) of aniline, 0.005 mmol (1.29 mg) of catalyst AgOTf, 3-phenyl-1-p-tolyl-?? Acrylketone 0.5 mmol (11.1 mg), toluene 2mL. at 100 o React in a C oil bath for 7 hours, cool to room temperature, extract the product with ethyl acetate, concentrate under reduced pressure, and purify the product by column chromatography to obtain a white solid product with a yield of 89%. 1 H NMR (500 MHz, CDCl 3 ) ppm: 8.36 (d, J =8.0 Hz, 1H), 8.25 (d, J = 8.0 Hz, 2H), 7.95 - 7.93 (d, J = 8.0 Hz, 1H), 7.85 (s, 1H), 7.81 - 7.74 (m, 1H), 7.61 - 7.52 (m, 6H), 7.38 - 7.36 (d, J = 8.0 Hz, 2H), 2.46 (s, 3H); 13 C NMR (500 MHz, CDCl 3 ): 156.9, 149.2, 148.6, 139.4, 138.4, 136.7, 120.2, 129.6, 129.5, 128.5, 128.2, 127.4, 126.1, 125.6, 125.5, 119.1, 21.5) C CalMS for E 22 h 17 N: [M + ], 295.1369; Found: 295.1365.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com