Sustained release preparation of isothiocyanate compound
A technology of ester compounds and isothiocyanic acid, applied in the direction of non-active ingredients of polymer compounds, active ingredients of esters, medical preparations of non-active ingredients, etc., can solve the problem of making stable and commercially available sustained-release preparations , Poor formability and solubility, easy to volatilize, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0131] The present invention also provides a preparation method of a sustained-release preparation of an isothiocyanate compound or a derivative thereof, the method comprising the following steps:
[0132] Provide isothiocyanate compounds or their derivatives;
[0133] Melting the sustained-release material and mixing it with the isothiocyanate compound or its derivatives to form a first dispersion;
[0134] The first dispersion is mixed with other excipients and prepared into a formulation.
[0135] The isothiocyanate compounds or their derivatives can be prepared by conventional methods, or obtained through commercial channels.
[0136] In another preferred example, the preparation is prepared by the following method:
[0137] The active ingredients are mixed and melted with biodegradable polymer materials, and then extruded into strips through a porous device. The strips are generally about 1mm in diameter. After being cut into lengths containing a single dose of medicine...
Embodiment 1
[0143] Embodiment 1: Excipient compatibility test
[0144] Method: Isothiocyanate compounds and auxiliary materials are mixed in a certain proportion (methods include simple mixing, melting, etc.). A blank group of auxiliary materials is set in parallel for each auxiliary material. After preparation, put it into a glass container, seal it, and then place it at 60°C for inspection, and detect it on day 0, day 5, and day 10.
[0145] Determination conditions: HPLC-UV method is used to determine the content of isothiocyanate compounds in the mixture, and the impurities in the process are monitored.
[0146] result:
[0147] Some of the data are as follows (investigated with phenylethyl isothiocyanate as the target):
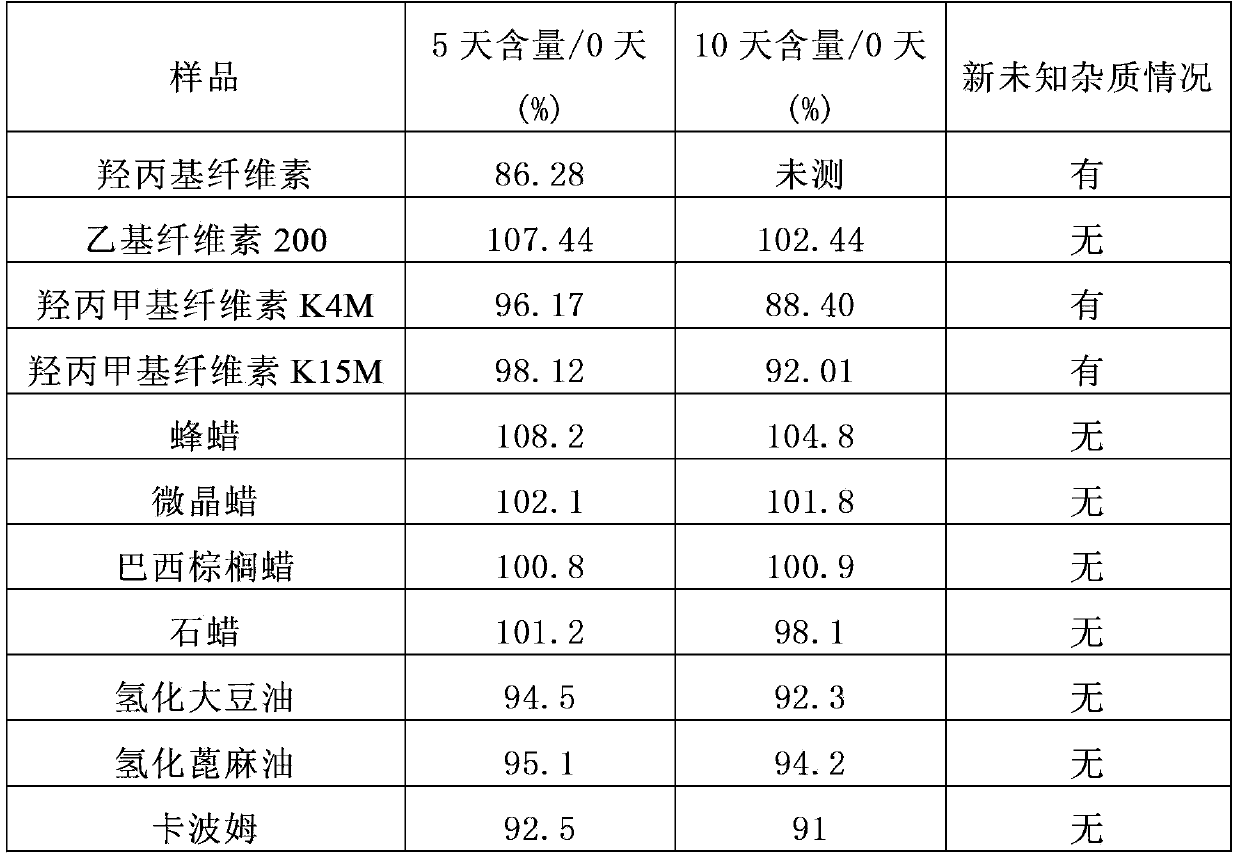
[0148] The content change of phenylethyl isothiocyanate in the different auxiliary materials of table 1
[0149]
[0150] From the data in the above table, it can be seen that the compatibility of the commonly used sustained and controlled release materials h...
Embodiment 2
[0152] Embodiment 2: auxiliary material stability experiment
[0153] On the basis of the excipient compatibility test in Example 1, the stabilizing effect of different excipients on isothiocyanate compounds was further investigated.
[0154] Method: Melt or grind the excipients and isothiocyanate compounds in a certain proportion and mix them evenly, put them in a vial, expose them at 60°C, and measure the isothiocyanate in the mixture by HPLC-UV method at different time points content of esters.
[0155] The results are shown in Table 2-Table 3.
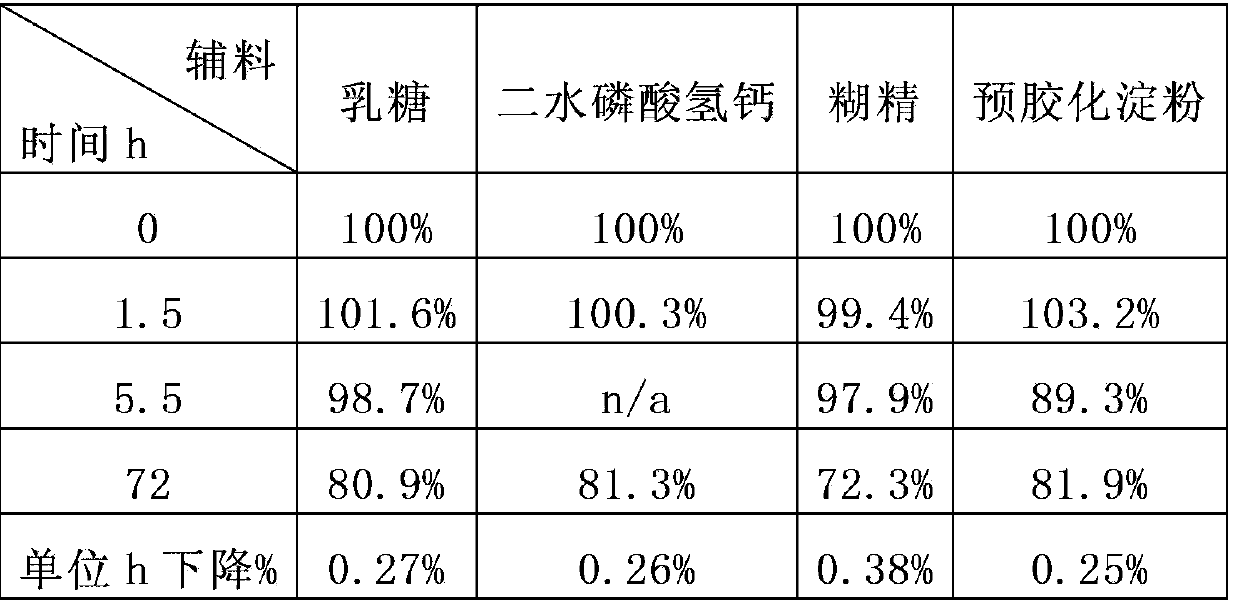
[0156] Table 2
[0157]
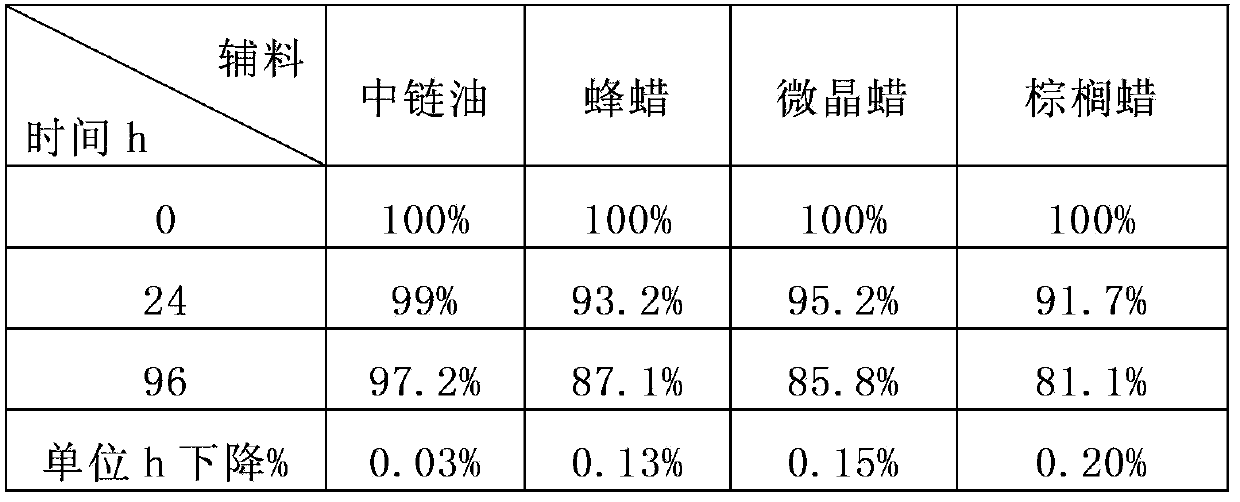
[0158] table 3
[0159]
[0160] The results of Tables 2 and 3 suggest:
[0161] Different auxiliary materials have a protective effect on the stability of isothiocyanate compounds under the test conditions, and medium chain oil> beeswax, microcrystalline wax, carnauba wax> other auxiliary materials; In terms of additives, wax or oil excipients have significant advantages in inhibiting the decli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com