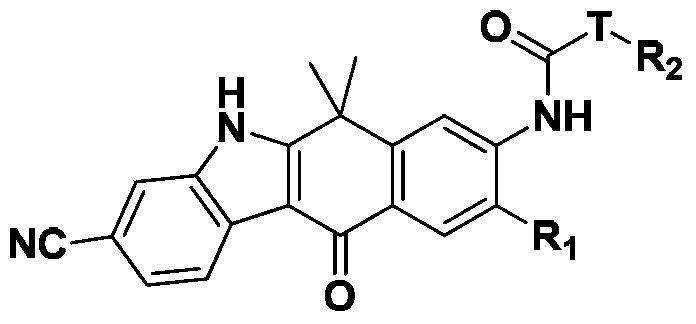
Amido substituted indolonaphthalenone derivatives and medicinal uses thereof
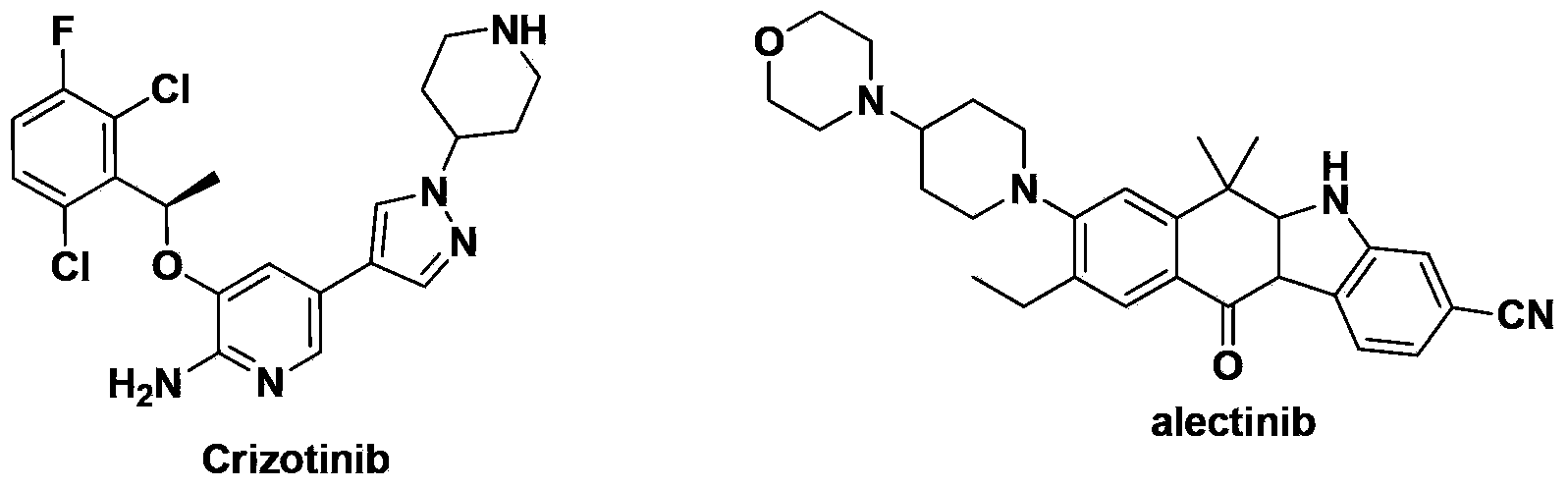
A substituent and unsubstituted technology, which is applied in the field of amido-substituted indoxaphthalenone derivatives and their medical applications, can solve the problems of Crizotinib bioavailability to be improved, Crizotinib drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
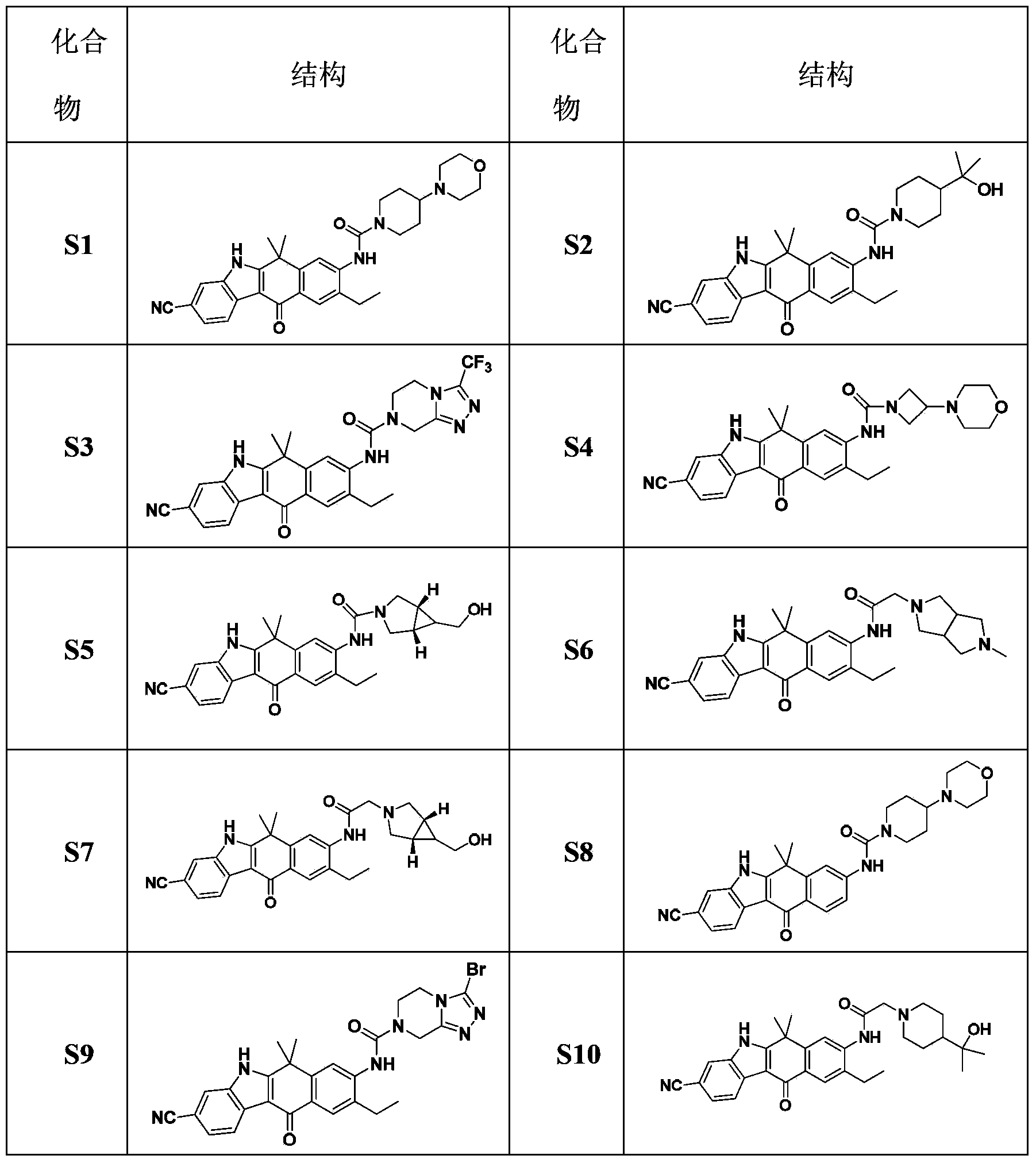
preparation Embodiment 1
[0037] Preparation of Example 1 Compound S1
[0038]
[0039] For the synthesis of compound 1-1, refer to CN102459172.
[0040] Synthesis of compound 1-2:
[0041] Dissolve compound 1-1 in ethanol, add 0.2eq cuprous iodide (CuI) and 0.2eq iron triacetylacetonate (Fe(acac) 3 ) After the addition, prepare an aqueous solution of sodium hydroxide and ammonia, dissolve 2eq of sodium hydroxide in 10eq of aqueous ammonia, add the aqueous solution of sodium hydroxide to the reaction solution, and seal the tube at 90°C for 48 hours. After the reaction was completed, the reaction solution was filtered, extracted with chloroform and water, the organic phase was mixed with silica gel and loaded on the column, CHCl 3 :MeOH:NH 3 .H 2 O=100:1:1~100:20:1 (volume ratio) to obtain compound 1-2.
[0042] 1 H NMR (300MHz, CDCl 3 )δ10.19(s,1H),8.51(d,J=8.1Hz,1H),8.14(s,1H),7.67(s,1H),7.50(dd,J=8.2,1.4Hz,1H), 6.81 (s, 1H), 4.15 (s, 2H), 2.55 (q, J=7.4Hz, 3H), 1.80 (s, 6H), 1.37–1.25 (m, ...
preparation Embodiment 2
[0046] Preparation of Example 2 Compound S2
[0047]
[0048] Synthesis of compound S2:
[0049] Compound S2 was synthesized the same as compound S1 except that compound 2-(4-piperidinyl)-2-propanol was used instead of 4-(4-piperidinyl)morpholine.
[0050] 1 H NMR (300MHz, CDCl 3 +MeOD)δ8.30(dd,J=8.2,0.7Hz,1H),8.04(s,1H),7.97(s,1H),7.65(dd,J=1.4,0.7Hz,1H),7.38(dd ,J=8.2,1.4Hz,1H),4.06(d,J=13.1Hz,2H),2.79(t,J=11.8Hz,2H),2.57(q,J=7.5Hz,2H),1.76(d ,J=11.6Hz,2H),1.63(s,6H),1.22(m,5H),1.06(s,6H).
preparation Embodiment 3
[0051] Preparation of Example 3 Compound S3
[0052]
[0053] Synthesis of compound S3:
[0054] Instead of 4-(4-piperidine Except for base) morpholine, the synthesis of compound S3 is the same as that of compound S1.
[0055] 1 H NMR (300MHz, CDCl 3 +MeOD)δ8.36(d,J=8.2Hz,1H),8.16(s,1H),7.73(s,1H),7.72(s,1H),7.42(dd,J=8.2,1.4Hz,1H ),4.99(s,2H),4.23(t,J=5.2Hz,2H),4.02(t,J=5.3Hz,2H),2.65(q,J=7.5Hz,2H),1.68(s,6H ),1.23(t,J=7.5Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com