Temozolomide suppository content measuring method by utilization of reversed-phase ion-pair chromatography method
A technology of temozolomide suppository and temozolomide, which is applied in the field of medicine and can solve the problems of inability to accurately obtain the actual content of the medicine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation of embodiment 1 temozolomide suppository
[0072] prescription:
[0073]
[0074] Weigh the temozolomide raw materials and auxiliary materials according to the prescription amount, heat to melt, and stir evenly; inject the prepared medicinal liquid into the suppository mold, the medicinal liquid slightly overflows the mold, cool; cut off the overflowing part, demould, and pack.
Embodiment 2
[0075] The detection of embodiment 2 temozolomide suppositories
[0076] (1) Preparation of reference solution
[0077] Accurately weigh an appropriate amount of temozolomide reference substance, dissolve and dilute it into a solution containing 0.05 mg of temozolomide in every 1 ml, and obtain the product.
[0078] (2) Preparation of the test solution
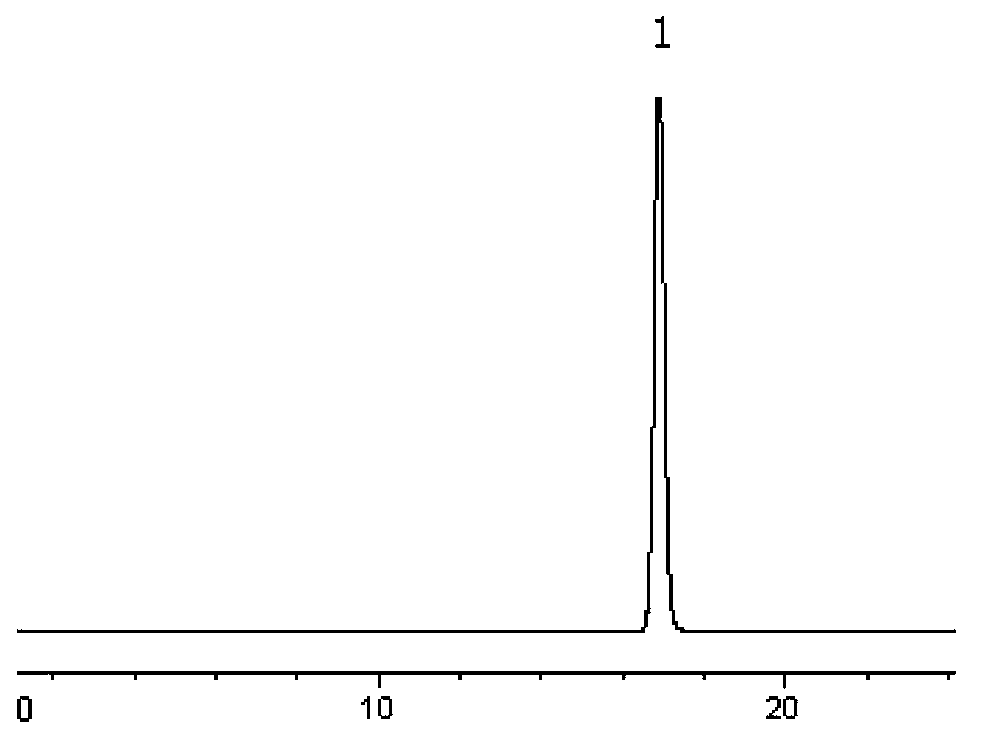
[0079] Take 10 capsules of the test product, accurately weigh them, chop them up, mix them evenly, accurately weigh an appropriate amount (about 50 mg of temozolomide), put them in a 100ml volumetric flask, add acetonitrile to the mark, and shake in a 45°C water bath to make temozolomide Dissolve, shake on the shaker for 2 minutes, then freeze in the refrigerator (-18°C) for 1 hour and filter immediately, accurately measure 1ml of the filtrate that has been brought to room temperature, put it in a 10ml volumetric flask, dilute to the mark with mobile phase, and shake Evenly, filter through a 0.45μm microporous membrane, take...
Embodiment 3
[0081] The detection of embodiment 3 temozolomide suppositories
[0082] (1) Preparation of reference solution
[0083] Precisely weigh an appropriate amount of temozolomide reference substance, dissolve and dilute it into a solution containing 0.025 mg of temozolomide in every 1 ml, and obtain the product.
[0084] (2) Preparation of the test solution
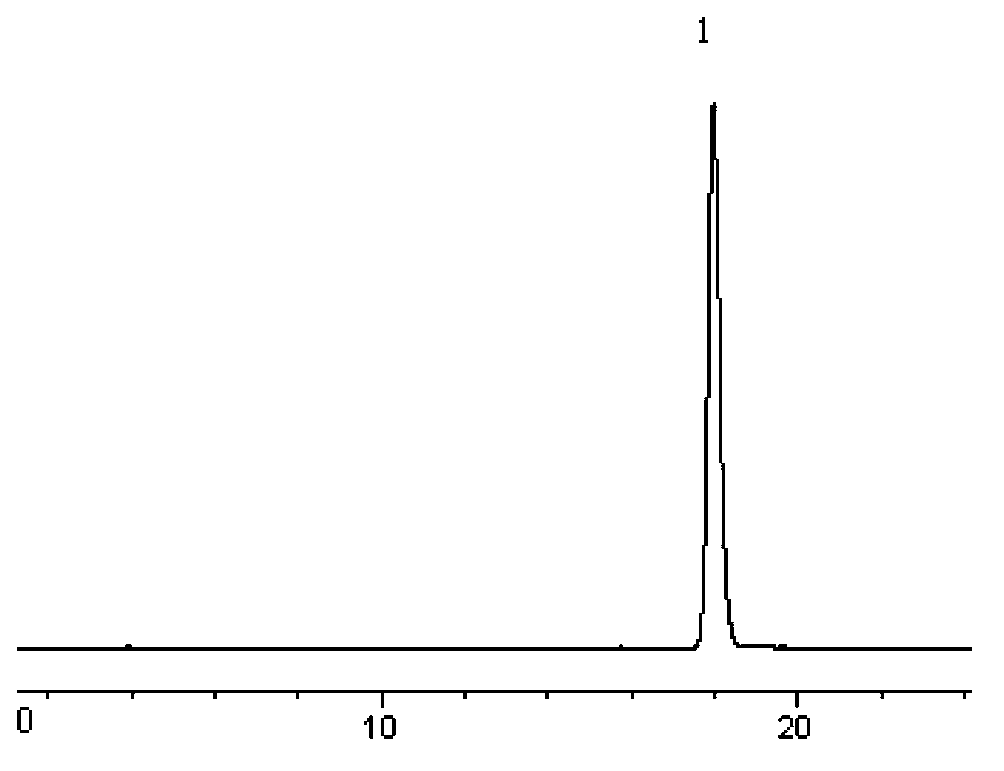
[0085] Take 10 capsules of the test product, accurately weigh them, chop them up, mix them evenly, accurately weigh an appropriate amount (about 50 mg of temozolomide), put them in a 100ml volumetric flask, add acetonitrile to the mark, and shake in a 45°C water bath to make temozolomide Dissolve, shake on the shaker for 2 minutes, then freeze in the refrigerator (-18°C) for 1 hour and filter immediately, accurately measure 1ml of the filtrate that has been brought to room temperature, put it in a 10ml volumetric flask, dilute to the mark with mobile phase, and shake Evenly, filter through a 0.45μm microporous membrane, take...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com