Preparation of amorphous ticagrelor
A ticagrelor and amorphous technology, applied in the direction of organic chemistry, can solve the problems of ticagrelor amorphous and other problems, and achieve the effect of good stability and stable and controllable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
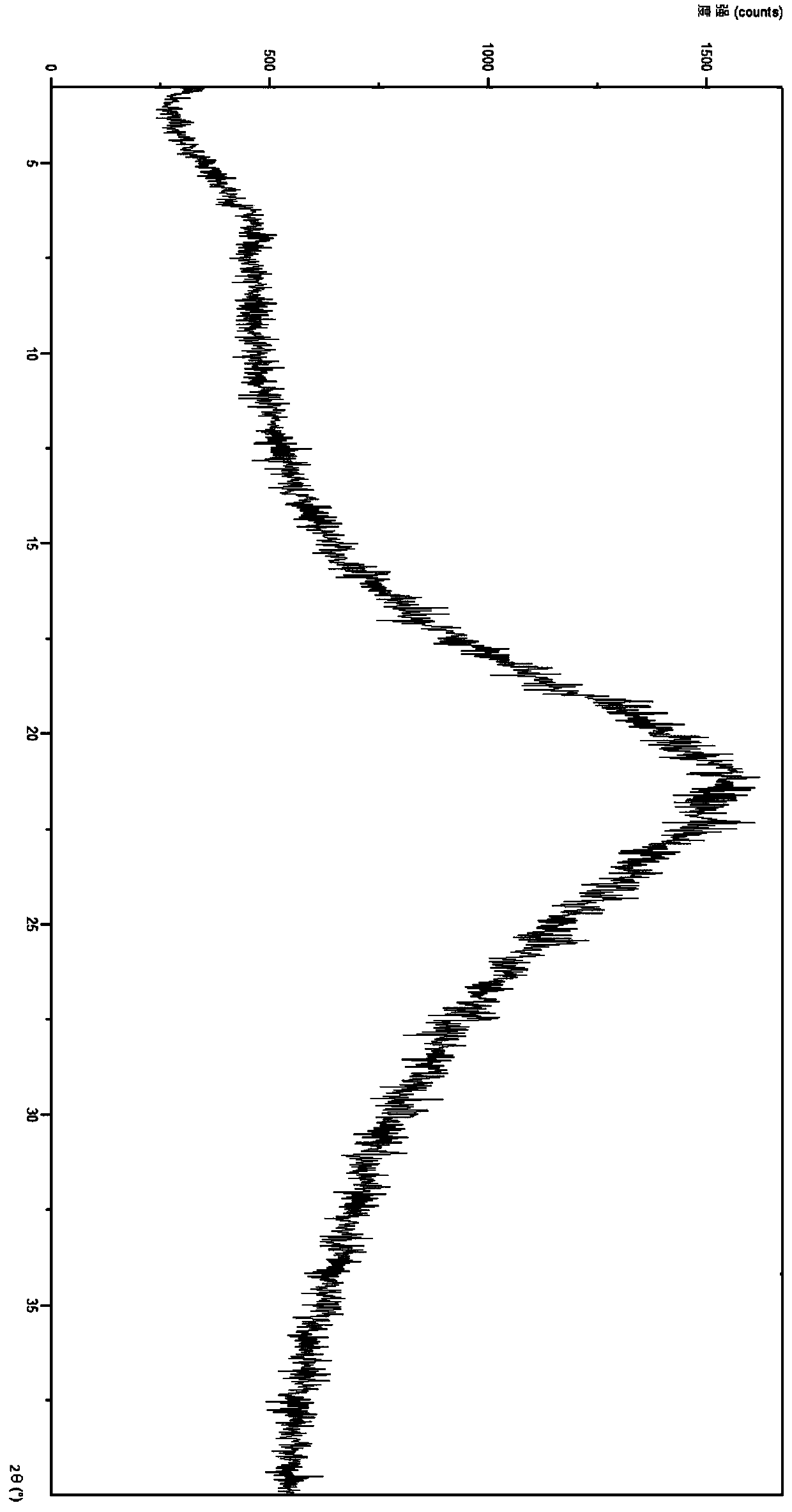
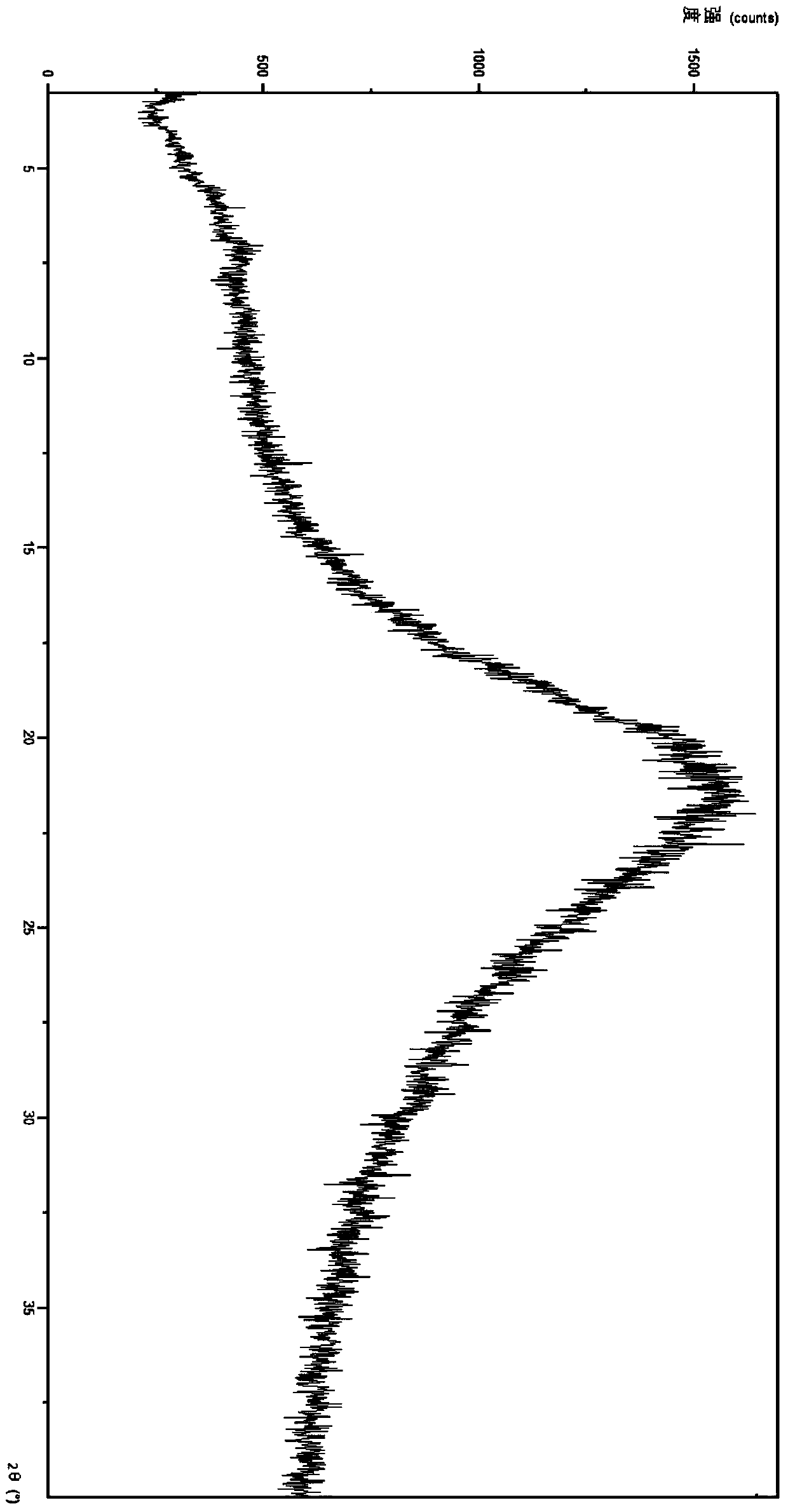
[0026] At room temperature, add 500 mg of crude ticagrelor into 30 mL of 1,4-dioxane, heat and stir at 55°C until dissolved. Rotate the above solution at about 50°C to remove about 40% of 1,4-dioxane; then pour the remaining solution into n-hexane (40ml) at about -10°C at one time, stirring at about 300rmp / min, a large amount of solids were precipitated, and filtered after about 5 minutes to obtain 530mg of solids, which weighed 460mg after 10 hours of alkaline drying at 40°C. Get a small amount of sample, use PANalytical Empyrean X-ray diffractometer to measure XRD, its X-ray powder diffraction pattern is as follows figure 1 shown.
Embodiment 2
[0028] In reaction vessel 1, 500 mg of crude ticagrelor was added into 20 mL of 1,4-dioxane, and heated to dissolve at 65°C. The above solution was rotated under reduced pressure at about 50°C to remove about 35% of 1,4-dioxane; in reaction vessel 2, n-hexane (40ml) was cooled to about -10°C, and about 5mg was added by Example 3 The freshly prepared ticagrelor is amorphous, and the stirring speed is adjusted to about 400rmp / min; then the remaining solution in reaction vessel 1 is poured into reaction vessel 2 at one time, a large amount of solid is precipitated, and it is filtered after about 5 minutes to obtain 550mg of solid, after 10 hours of alkaline autoclaving at 40°C, weighed 480.22mg of white solid. Get a small amount of solid, use PANalytical Empyrean X-ray diffractometer to measure XRD, its X-ray powder diffraction pattern is as follows figure 1 shown.
Embodiment 3
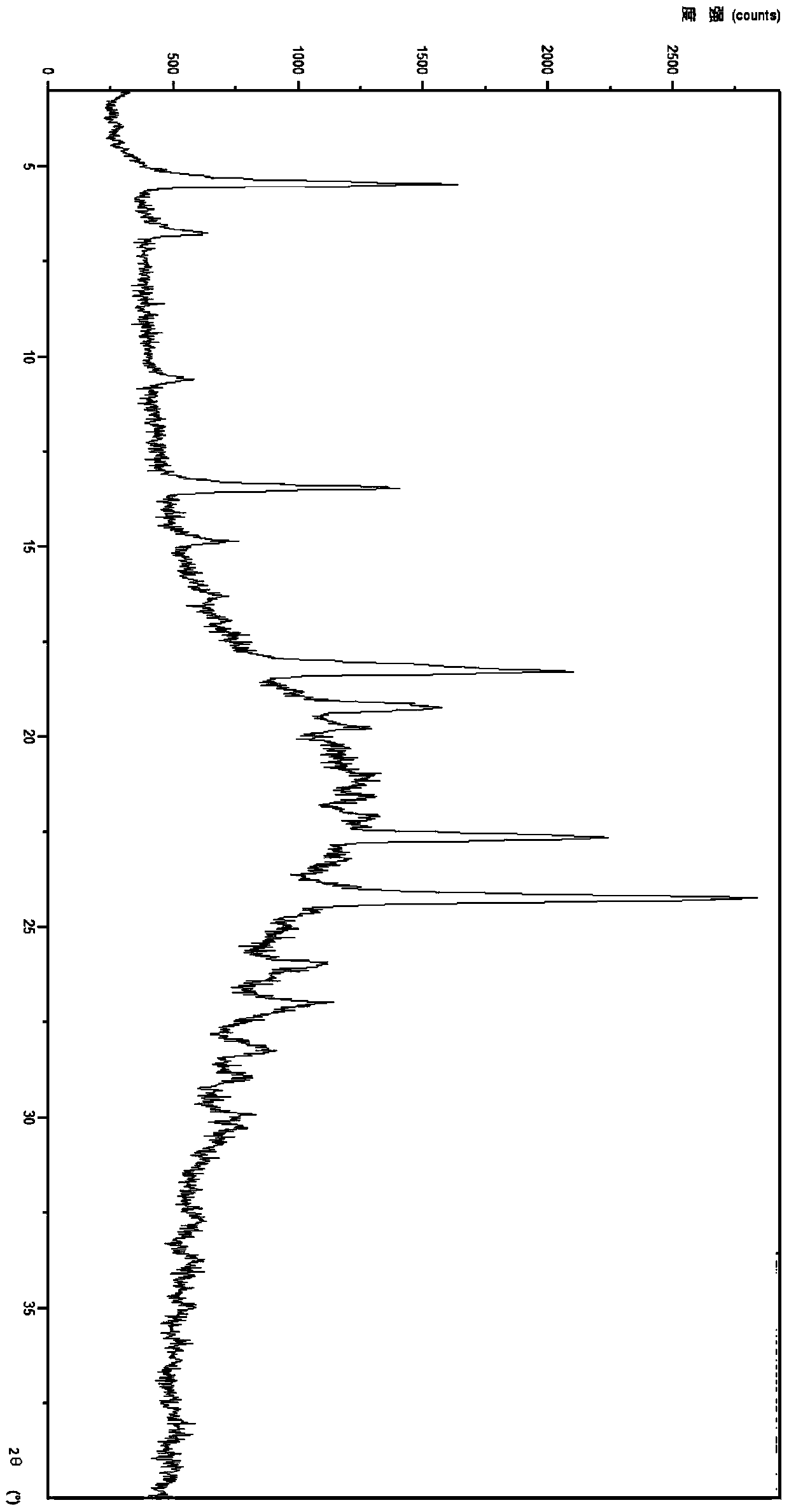
[0029] Example 3 Prepared according to the method disclosed in PCT application WO 2014 / 083139
[0030] At 20-25°C, 1g of ticagrelor was dissolved in 10ml of methanol to form a solution, and the formed solution was rotary evaporated under reduced pressure at a vacuum of 5mmHg, and the temperature was controlled at 45-50°C to obtain amorphous ticagrelor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com