Stable liquid lipid calibrator
A technology for calibrators and liquids, applied in material inspection products, biological tests, etc., can solve the problems of long stabilization time, high calibration results, and high price, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) The influence of different stabilizers on the stability of calibrators
[0039] Prepare the serum matrix liquid containing the test target apolipoprotein A-I and apolipoprotein B according to the following formula, add stabilizers of different concentrations into the matrix, and place it in a 37°C water bath for 16 hours, before and after the water bath. Use Ruiyuan Company to sell apolipoprotein A-I and apolipoprotein B detection kit (immunoturbidimetric method) and its calibrators, and detect the content of ApoA-I and ApoB in serum matrix fluid according to their respective instructions. Determine the change of the content to investigate the stability and analyze the effect of different stabilizers added. The four additives in Table 1 can all improve the stability of ApoA-I and ApoB in the serum matrix, and are positively correlated with the concentration of each stabilizer.
[0040] Serum matrix fluid containing ApoA-I and ApoB: pH7.4
[0041]
[0042]
[0043] Table ...
Embodiment 2
[0058] The difference between this embodiment and embodiment 1 is only: the lipid calibrator preparation part of the present invention:
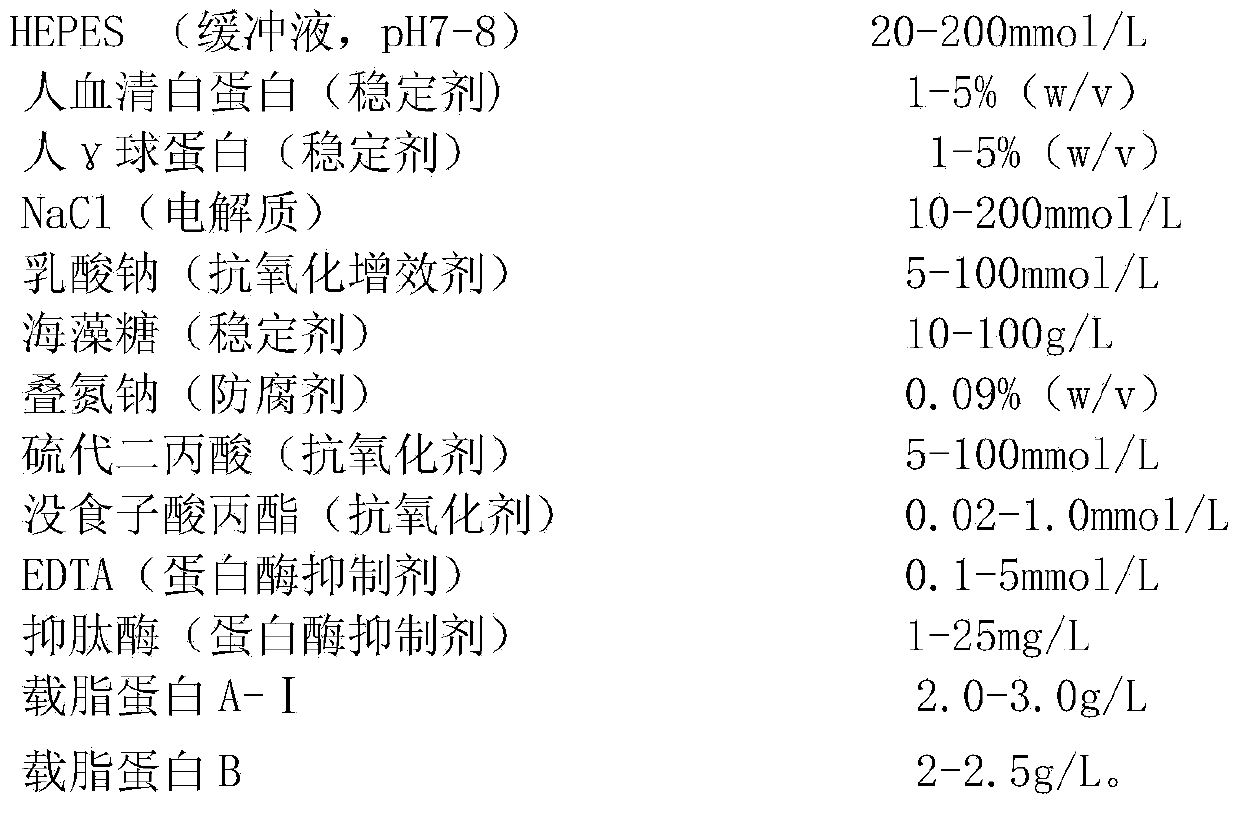
[0059] The lipid calibrator is a composite calibrator containing high-value apolipoprotein A-I and apolipoprotein B. The formula is as follows:
[0060]
[0061] In this example, after the standard product is refrigerated at 2-8°C for one year, the recovery rate of apolipoprotein A-I is greater than 93%, apolipoprotein B is greater than 92%, and the calibration product contains apolipoprotein. The calibration accuracy of protein A-I and apolipoprotein B content is good, and the similar serum matrix and additives in the calibrator do not interfere with the detection of apolipoprotein A-I and apolipoprotein B.
Embodiment 3
[0063] The difference between this embodiment and embodiment 1 is only: the lipid calibrator preparation part of the present invention:
[0064] The lipid calibrator is a composite calibrator containing high-value apolipoprotein A-I and apolipoprotein B. The formula is as follows:
[0065]
[0066] In this example, after the standard product is refrigerated at 2-8°C for one year, the recovery rate of apolipoprotein A-I is greater than 93%, apolipoprotein B is greater than 92%, and the calibration product contains apolipoprotein. The calibration accuracy of protein A-I and apolipoprotein B content is good, and the similar serum matrix and additives in the calibrator do not interfere with the detection of apolipoprotein A-I and apolipoprotein B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com