Application of L-arabinose to preparation of medicine or health care products for preventing or curing hyperammonemia
A technology of arabinose and hyperammonemia, which is applied in the application field of medicine or health products, and can solve problems such as hyperammonemia that have not been found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
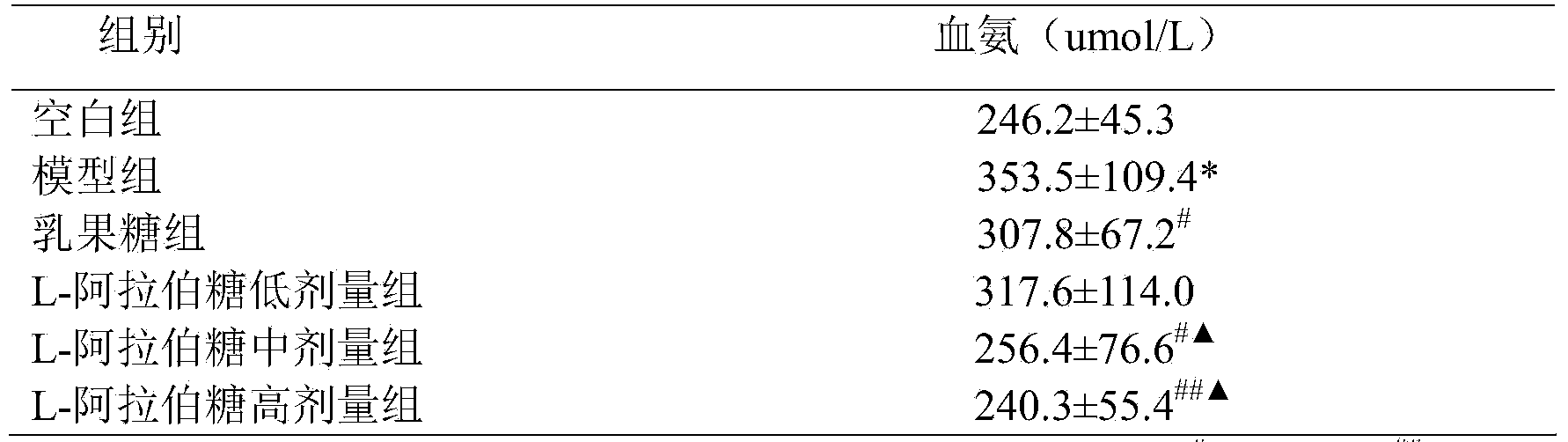
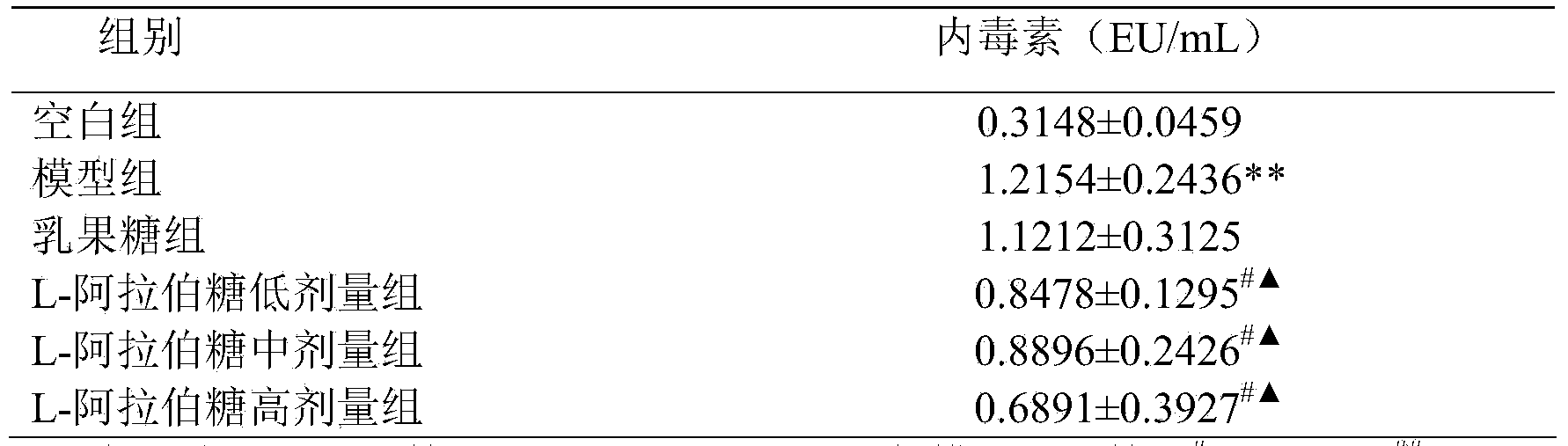
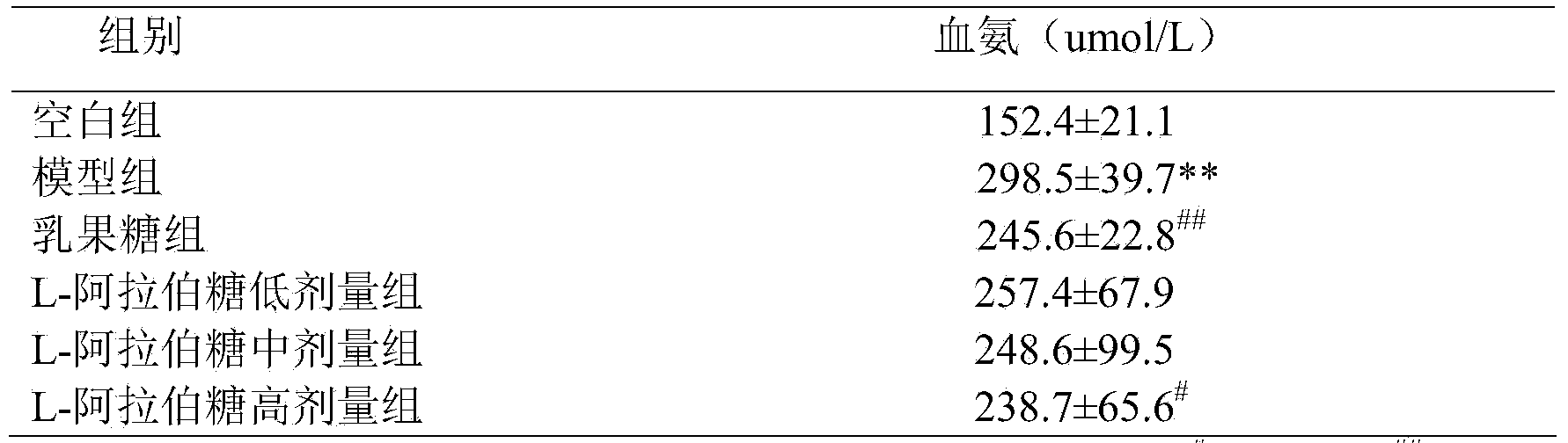
[0009] The effect of reducing blood ammonia and endotoxin of L-arabinose will be further illustrated through experiments below.
[0010] 1. L-arabinose to CCl 4 Effect of blood ammonia in mice with liver injury
[0011] 1. Experimental materials and methods
[0012] 1.1 Drugs and reagents
[0013] L-arabinose (purity 99%): Tang Chuan Biotechnology (Xiamen) Co., Ltd., batch number: 1009081; Lactulose oral solution (66.7%): Beijing Hanmei Pharmaceutical Co., Ltd., batch number: 130457; blood ammonia determination kit, Nanjing Jiancheng Bioprocess Research Institute; Chromogenic Limulus Reagent Kit (quantitative detection of endotoxin), Xiamen Limulus Reagent Experimental Factory Co., Ltd.; the rest of the reagents are domestic analytical grade.
[0014] 1.2 Instruments
[0015] TGL-16R high-speed refrigerated centrifuge Zhuhai Heima Company; RT-9100 semi-automatic biochemical analyzer Shenzhen Leidu Life Science Co., Ltd.; electronic balance, Mettler-Toledo Instruments Shang...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com