Method for determining content of mythyl p-hydroxybenzoate and sodium benzoate in solution
A technology of methyl hydroxybenzoate and sodium benzoate, applied in the field of chemical inspection, can solve the problems of increased workload of inspection personnel in the deployment and use of analytical instruments, cumbersome inspection methods, passive work arrangements, etc., and achieves easy operation, good stability, and economical efficiency. effect of personnel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
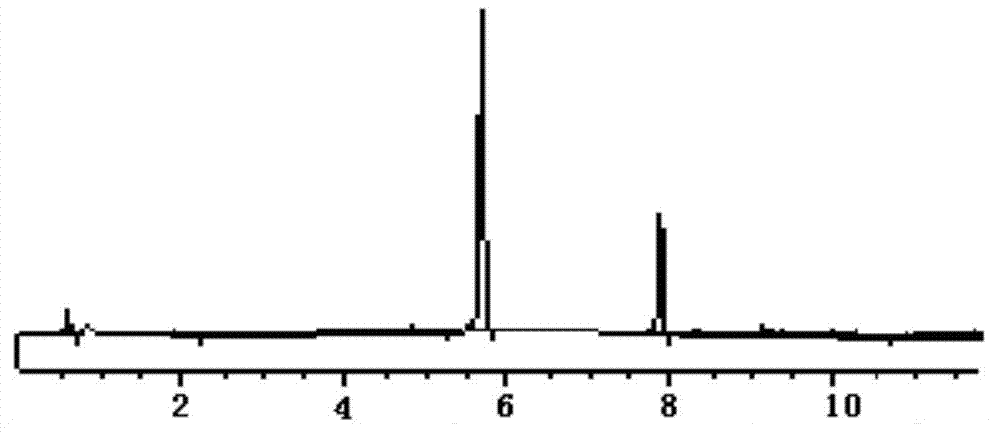
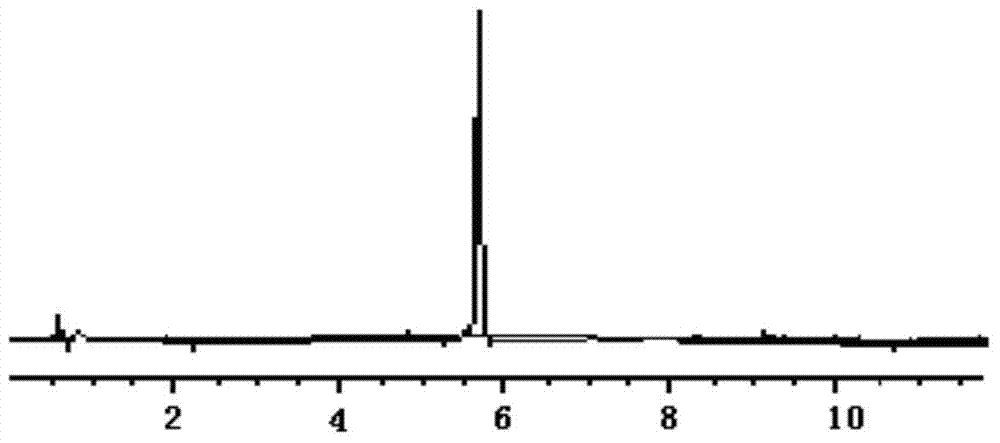
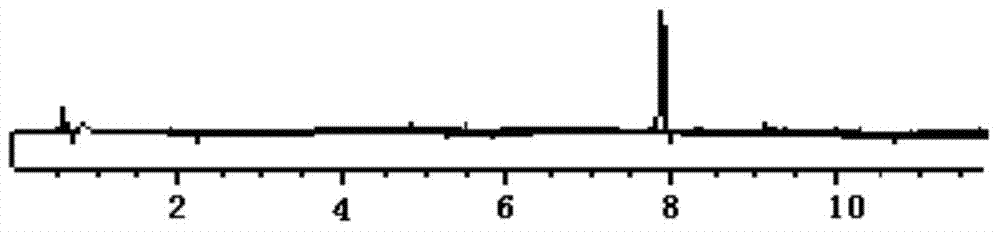
[0022] According to the "Chinese Pharmacopoeia" 2010 edition two appendix VD high performance liquid chromatography determination.
[0023] Experimental method and process:
[0024] 1. Instruments and reagents
[0025] Chromatograph: 1260 high performance liquid chromatograph (manufacturer: Agilent)
[0026] Analytical balance: AG285 type (manufacturer: Mettler)
[0027] Test product: a drug preparation to be tested, known to contain 0.5 mg / ml of methyl p-hydroxybenzoate and 1 mg / ml of sodium benzoate.
[0028] 2. Chromatographic conditions and system adaptability
[0029] Chromatographic column Shim-pack VP-ODS C18 column (4.6mm×250mm, 5μm); with 1% acetic acid-acetonitrile as the mobile phase, the volume ratio of the aqueous phase to the organic phase is 70:30, the detection wavelength is 230nm, and the flow rate is 1.0ml per minute, the column temperature is 30°C, the number of theoretical plates is not less than 3000 based on sodium benzoate, and the resolution should ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com