Novel herbicidal active pyridine salicylic acid compound, prepare method thereof, and purpose of being herbicidal
A pyrimidine salicylic acid, herbicidal activity technology, applied in the field of 2,6-bis(oxy) benzoic acid imido ester derivatives, can solve the problems of narrow herbicidal spectrum, low herbicidal performance, poor selectivity, etc. The effect of good purity, environmental friendliness and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
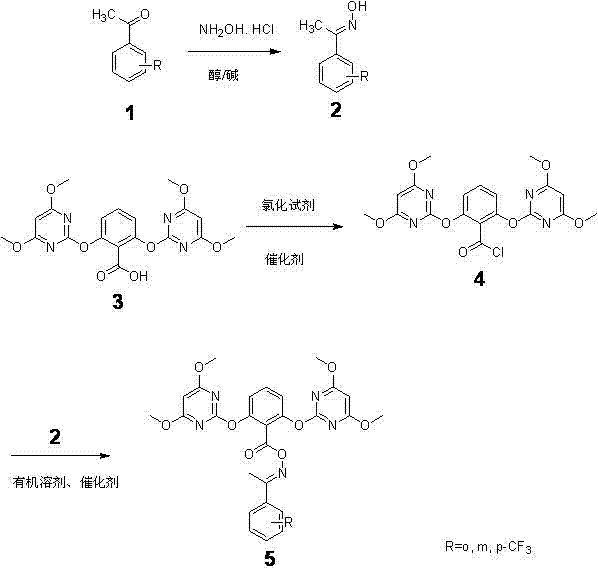
[0044] Embodiment 1: Preparation of 2-trifluoromethylacetophenone oxime
[0045] Add hydroxylamine hydrochloride (8.3 g, 120 mmol), methanol (150 ml), 20% aqueous sodium hydroxide solution (24.0 g, 120 mmol), 2-trifluoromethylacetophenone (17.4 g, 100 mmol), reacted under nitrogen protection at 25°C for 3 hours, after the reaction results, distilled off methanol under reduced pressure, added water, filtered to obtain 18.1 g of solid 2-trifluoromethyl acetophenone oxime, yield 89.1%, Melting point: 110.5-112.5°C.
Embodiment 2
[0046] Example 2: O-[2,6-bis(4,6-dimethoxypyrimidinyl-2-yloxy)benzoyl](2-trifluoromethyl)acetophenone oxime (HP-001) preparation of
[0047] Add 2,6-bis(4,6-dimethoxy-2-pyrimidinyl-2-oxyl)benzoic acid (21.5 g, 50 mmol), oxalyl chloride (12.7 g, 100.0 mmol) into a 500 ml reaction flask , 0.5 milliliters of N, N-dimethylformamide, 250 milliliters of dichloromethane, heated and refluxed for 8 hours, after the reaction was completed, the excessive oxalyl chloride and the organic solvent dichloromethane were evaporated under vacuum and reduced pressure, cooled to room temperature, and dichloromethane was added Chloromethane 300 ml, 2-trifluoromethylacetophenone oxime (10.2 g, 50.2 mmol), DMAP (0.60 g, 5 mmol), under the protection of nitrogen, react at room temperature for 10 hours, after the reaction results, the reaction solution is Concentrate under reduced pressure to recover dichloromethane, and recrystallize with methanol to obtain white crystals of O-[2,6-bis(4,6-dimethox...
Embodiment 3
[0050] Embodiment 3: the preparation of 3-trifluoromethyl acetophenone oxime
[0051] Add hydroxylamine hydrochloride (6.9 g, 100 mmol), ethanol (250 ml), 20% aqueous sodium hydroxide solution (20.0 g, 100 mmol) and 3- Trifluoromethylacetophenone (17.4 g, 100 mmol) was reacted under nitrogen protection at 25°C for 2 hours. After the reaction was completed, methanol was distilled off under reduced pressure to obtain a solid 3- Trifluoromethyl acetophenone oxime 18.5g, yield 90.6%. Melting point: 79.6~81℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com