Self-discharge detection method of lithium iron phosphate battery
A lithium iron phosphate battery and detection method technology, applied in the direction of measuring electricity, measuring devices, measuring electrical variables, etc., can solve the problems that cannot reflect the actual situation of a single battery, require high parameter accuracy, and take a long time to test. Accurate and effective, improve product quality, eliminate the effect of polarization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
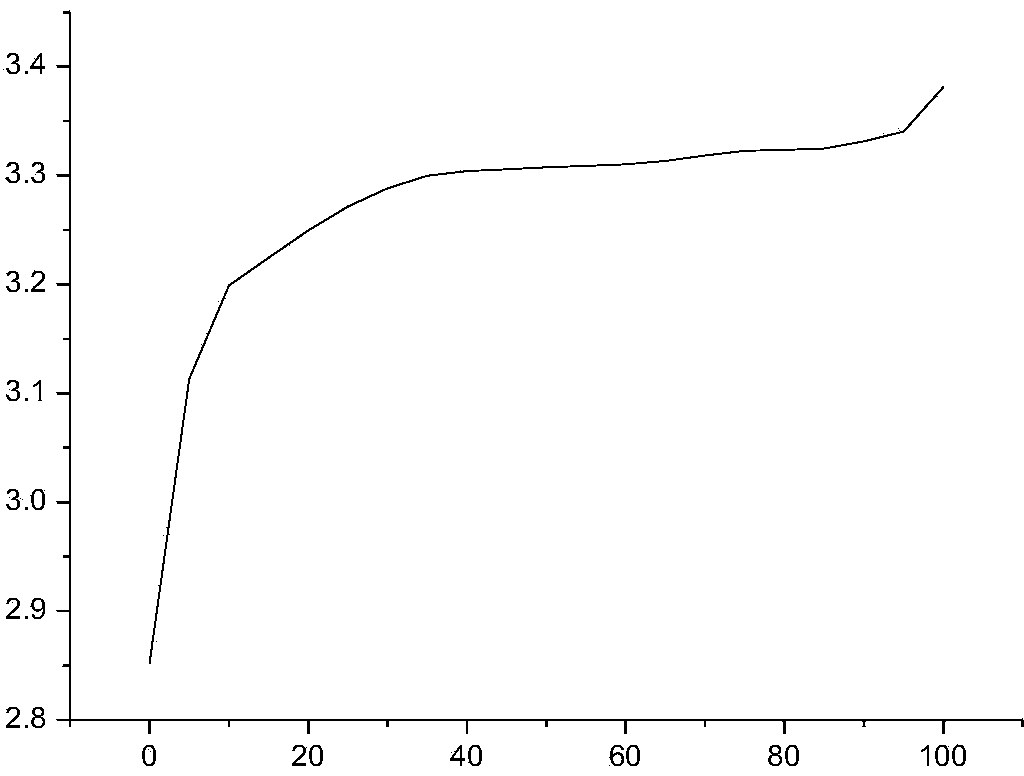
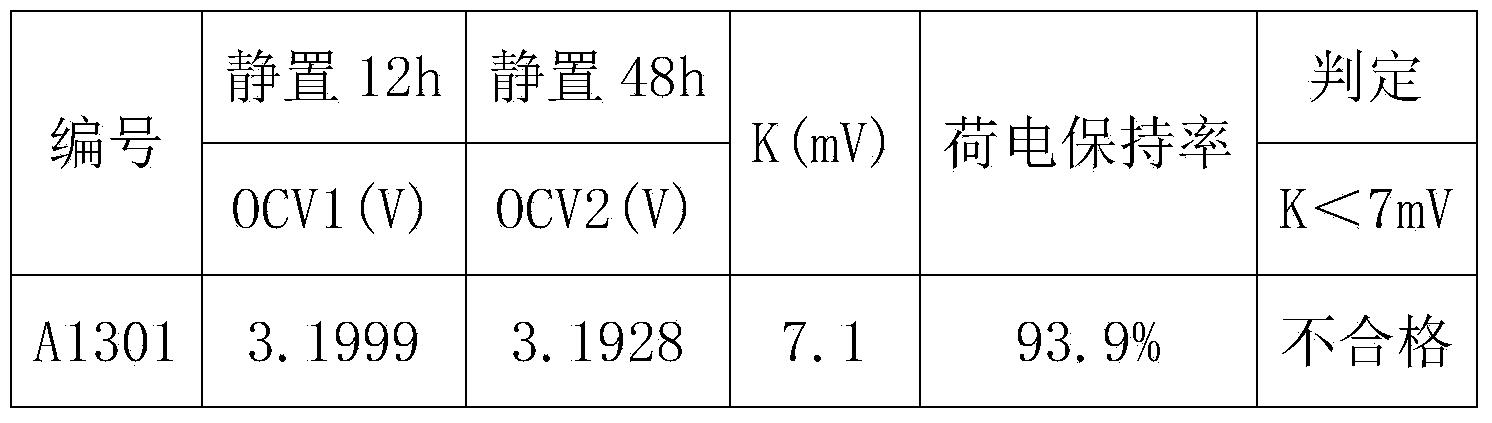
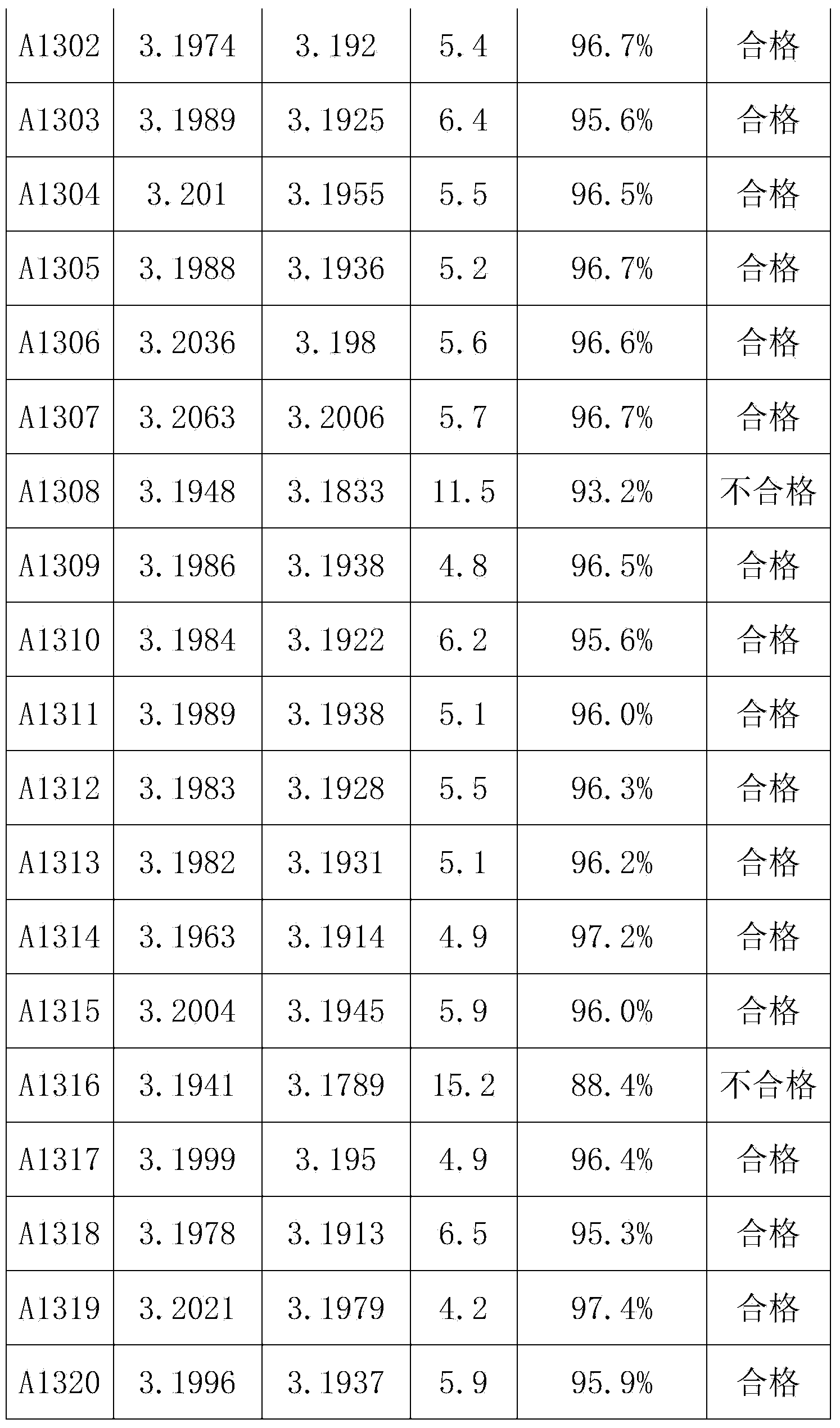
[0040] Taking a lithium iron phosphate battery with a capacity of 100AH as an example, first charge and discharge the lithium iron phosphate battery at a rate of 0.5C for 3 times, discharge to 2.5V at a rate of 0.3C, leave it for 30min, and then discharge to 2.5V at a rate of 0.05C. V; put it aside for 30 minutes, charge it to 3.21V with a constant current of 0.1C, let the battery stand at room temperature 20°C for 12 hours, test and record the open circuit voltage OCV 1 ;The battery was left standing at a high temperature of 50°C for 48 hours, and the open circuit voltage OCV was tested and recorded. 2 , calculate K=OCV 1 -OCV 2 After that, test the charge retention data of all the batteries in the group according to the GB / T743-2006 charge retention method, as shown in Table 1:
[0041]
[0042]
[0043] It can be concluded from Table 1 that when K>7.0mV, the charge retention rate of the battery is less than 94%, and when K<7.0mV, the charge retention rate of the bat...
Embodiment 2
[0045] Taking a lithium iron phosphate battery with a capacity of 100AH as an example, first charge and discharge the lithium iron phosphate battery at a rate of 0.5C for 3 times, discharge to 2.5V at a rate of 0.3C, leave it for 30min, and then discharge to 2.5V at a rate of 0.05C. V, put it aside for 30 minutes, charge it to 3.21V with a constant current of 0.1C, test and record the open circuit voltage OCV1 when the battery stands at room temperature 20°C for 12 hours; test and record the open circuit voltage OCV2 when the battery stands at a high temperature of 50°C for 84 hours; calculate Get K=OCV 1 -OCV 2 After that, all the batteries in the group are tested for the charge retention data according to the GB / T743-2006 charge retention method, as shown in Table 2:
[0046]
[0047]
[0048] It can be concluded from Table 2 that when K>10.0mV, the charge retention rate of the battery is less than 94%, and if K<10.0mV, the charge retention rate of the battery is gr...
Embodiment 3
[0050] Taking a lithium iron phosphate battery with a capacity of 100AH as an example, first charge and discharge the lithium iron phosphate battery at a rate of 0.5C for 3 times, discharge to 2.5V at a rate of 0.3C, leave it for 30min, and then discharge to 2.5V at a rate of 0.05C. V; put it aside for 30 minutes, charge it to 3.29V with a constant current of 0.1C, let the battery stand at room temperature 20°C for 12 hours, test and record the open circuit voltage OCV 1 ;The battery was left standing at a high temperature of 50°C for 48 hours, and the open circuit voltage OCV was tested and recorded. 2 , calculate K=OCV 1 -OCV 2 After that, test the charge retention data of all the batteries in the group according to the GB / T743-2006 charge retention method, as shown in Table 3:
[0051]
[0052]
[0053] It can be concluded from Table 3 that the voltage changes slowly in this state of charge, and the overall K value is small, which is easily disturbed by other fact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com