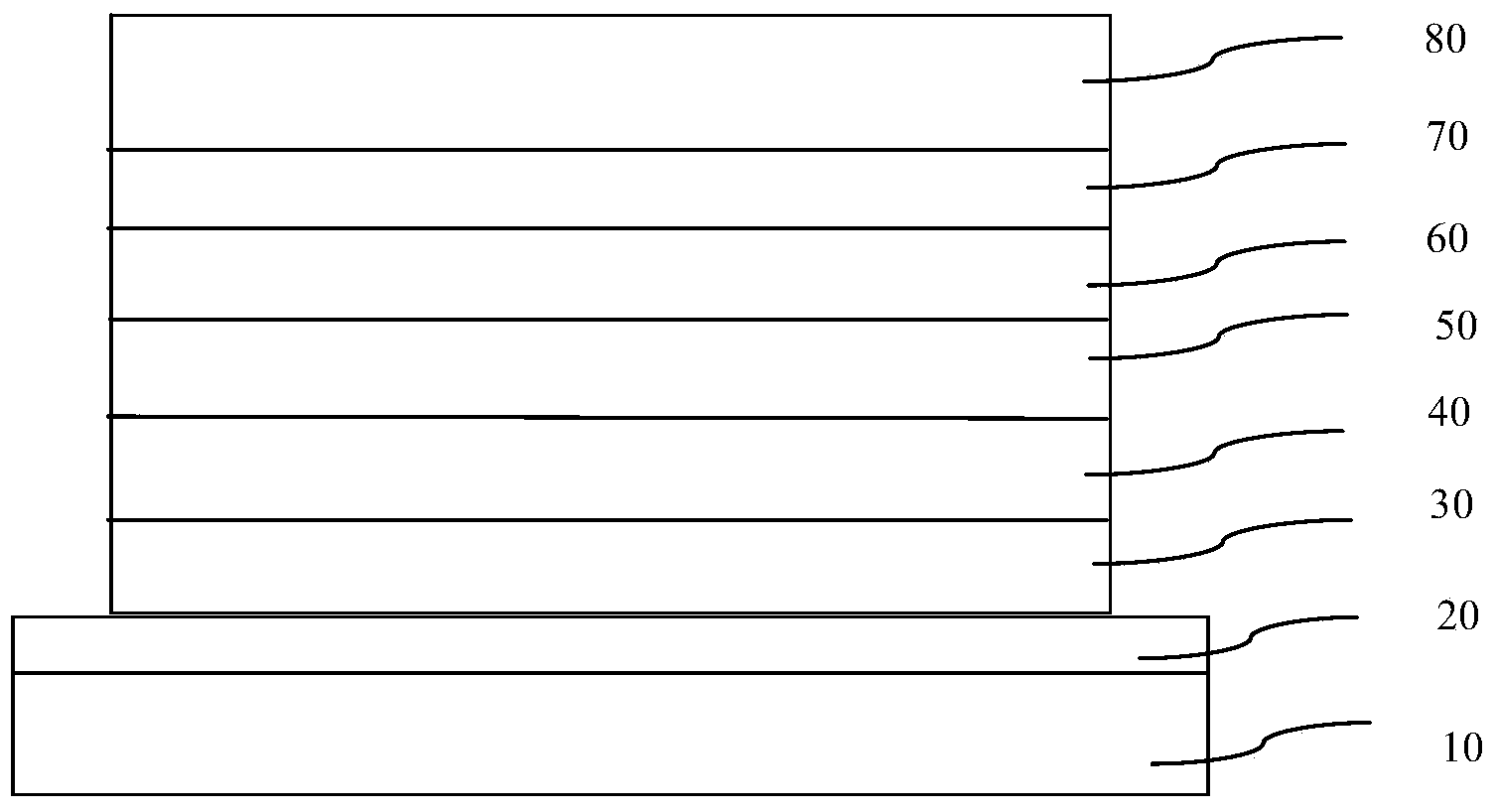
Organic electroluminescent material and organic electroluminescent device
An electroluminescent material and electroluminescent technology, applied in the direction of luminescent materials, electro-solid devices, electrical components, etc., can solve the problems of short lifespan of thermally stable devices and few materials, and achieve improved lifespan, high electron transmission and The effect of injection ability, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: compound ANP8 is synthesized
[0055]
[0056] Synthesis of intermediate 3
[0057] Add acenaphthylquinone (84g, 0.46mol), 1,3-diphenylacetone (72.8g, 0.34mol), 600ml ethanol, and 56g potassium hydroxide into a four-necked flask, start stirring, blow nitrogen gas, and reflux for 2 hours. Cool at room temperature, filter, and rinse the filter cake twice with ethanol to obtain 130 g of a black solid with a yield of 91%.
[0058] Synthesis of compound ANP8
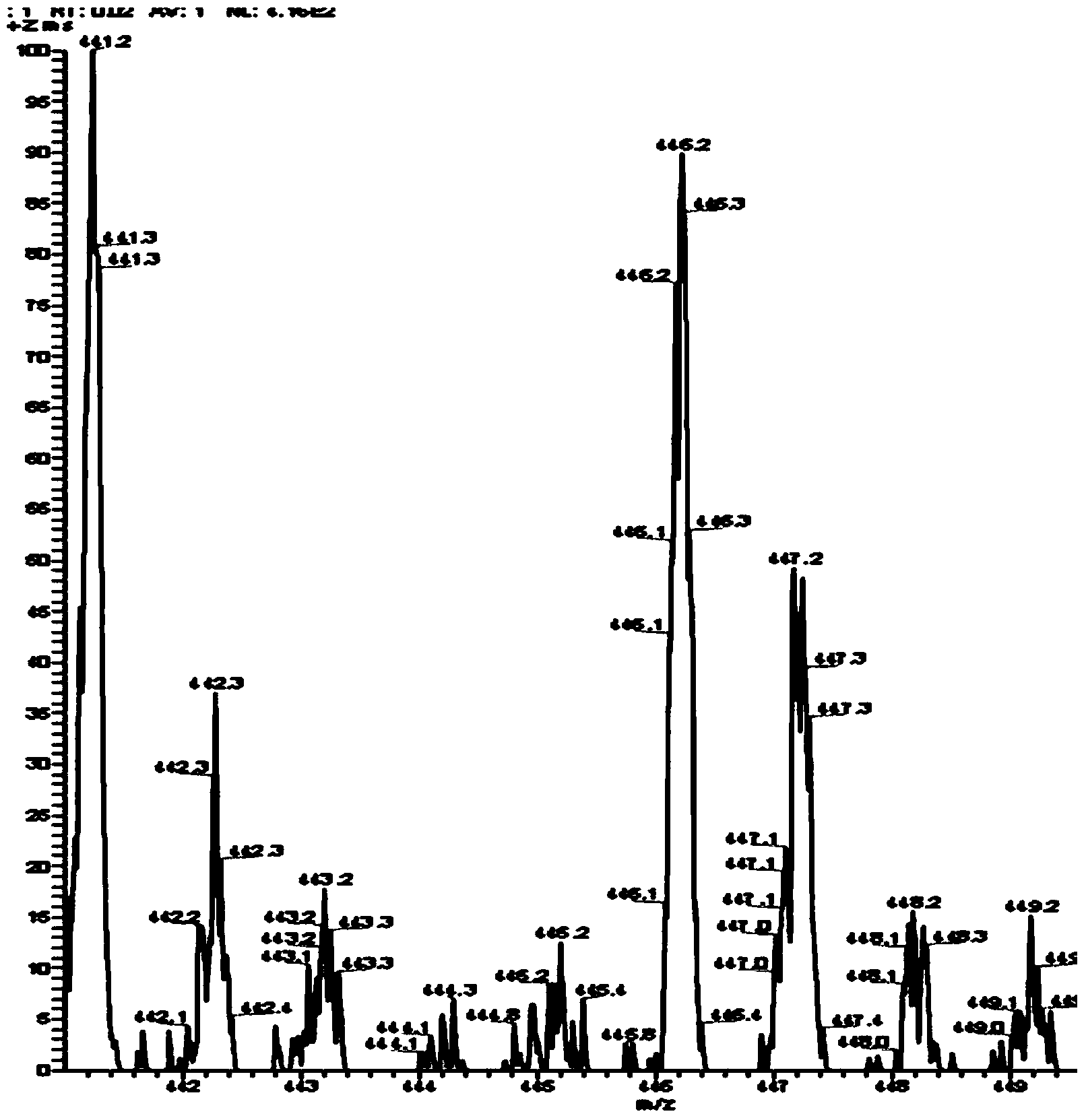
[0059] Intermediate 3 (3.56 g, 10 mmol) and Intermediate 4 (4.69 g, 40 mmol) were mixed under nitrogen and heated to reflux for 48 hours (external temperature 280° C.). The resulting brown solution was cooled to obtain a brown solid, which was crystallized from dichloromethane-acetone after passing through a silica gel column using petroleum ether as the eluent to obtain ANP 8 as white crystals. 0.23 g of product was obtained, yield 5%. ESI-MS m / s calculated value C 34 h 23 N: 445.18, measured va...
Embodiment 2
[0060] Embodiment 2: compound ANP34 is synthesized
[0061]
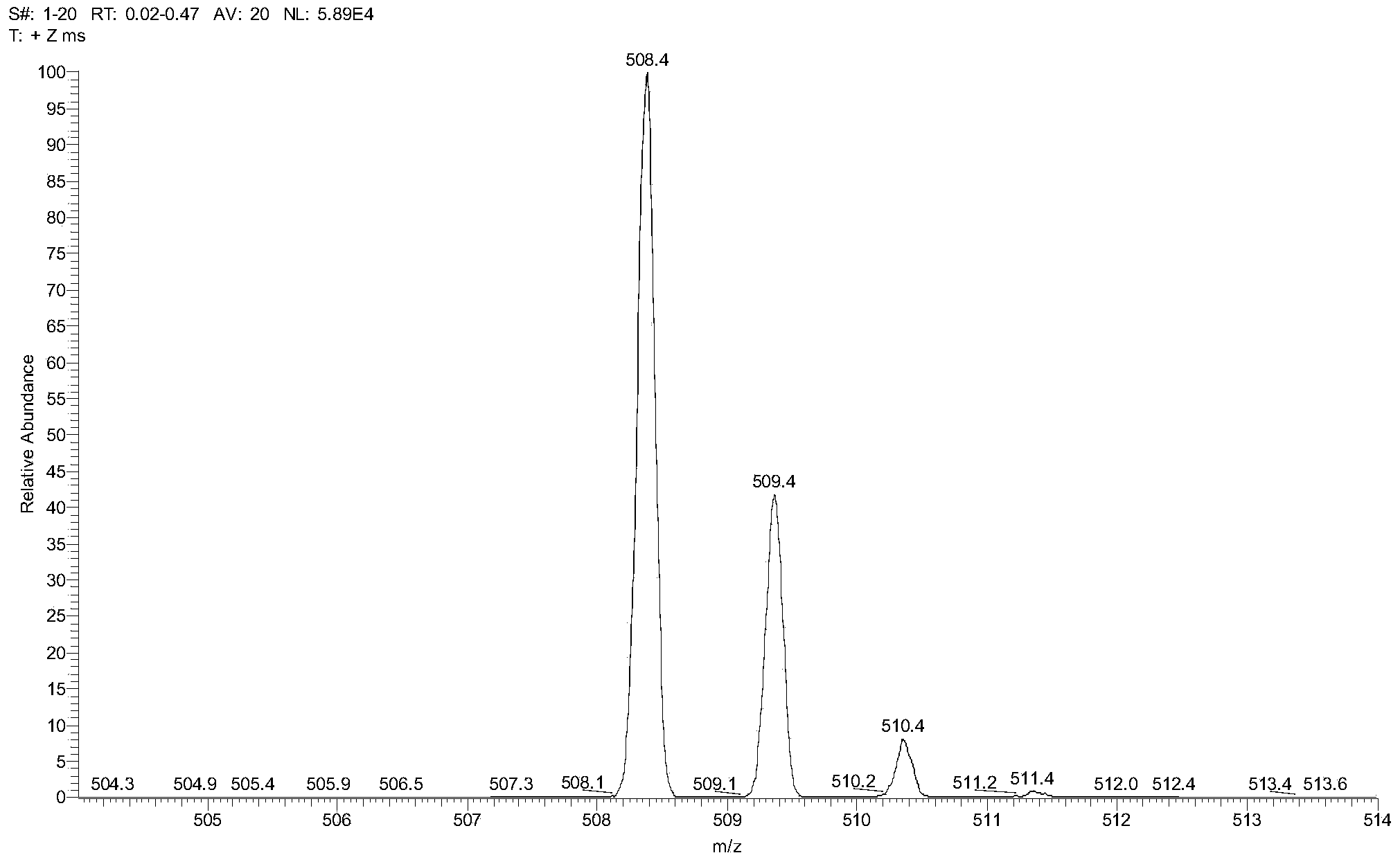
[0062] Intermediate 3 (3.56g, 10mmol) and Intermediate 6 (7.17g, 40mmol), 60ml of diphenyl ether were mixed under nitrogen and heated to reflux for 48 hours (external temperature 280°C). The resulting brown solution was cooled to obtain a brown solid, which was crystallized from dichloromethane-acetone after passing through a silica gel column using petroleum ether as the eluent to obtain ANP34 as light yellow crystals. 1.37 g of product were obtained, a 27% yield. 1 H NMR (400MHz, CDCl 3 ,δ): 7.98–7.95(m,2H), 7.90–7.82(m,2H), 7.65–7.31(m,20H), 6.93–6.89(d,1H). See Figure 5 . MALDI-TOF-MS m / s calculated value C 39 h 25 N: 507.20, measured value [M+H] + :508.50. See image 3
Embodiment 3
[0063] Embodiment 3: compound ANP64 is synthesized
[0064]
[0065] Intermediate 3 (3.56g, 10mmol) and Intermediate 8 (4.30g, 5mmol, synthesized according to Organic & Biomolecular Chemistry, 10(24), 4704-4711; 2012), 60ml of diphenyl ether were mixed and heated under nitrogen Reflux for 48 hours (external temperature 280°C). The resulting brown solution was allowed to cool to give a brown solid which was crystallized from dichloromethane-acetone to afford ANP64 as white crystals. 2.15 g of product were obtained, yield 50%. 1 H NMR (400MHz, CDCl 3 ,δ): 7.98–7.94 (m, 2H), 7.87–7.77 (m, 2H), 7.67–7.22 (m, 18H), 6.96 (d, 1H, J=10Hz), 1.23 (s, 6H). See Figure 6 . 13 C NMR (100MHz, CDCl 3 ,δ): 27.8, 47.1, 119.6120.3, 122.6, 123.6, 124.9, 125.3, 127.0, 127.3, 127.4, 127.9, 128.0, 128.1, 128.8, 129.0, 129.5, 130.0, 130.5, 130.9, 1343.2. See Figure 7 . ESI-MS m / z calculated value C 42 h 29 N: 547.23, measured value [M+H] + :548.53. See Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com