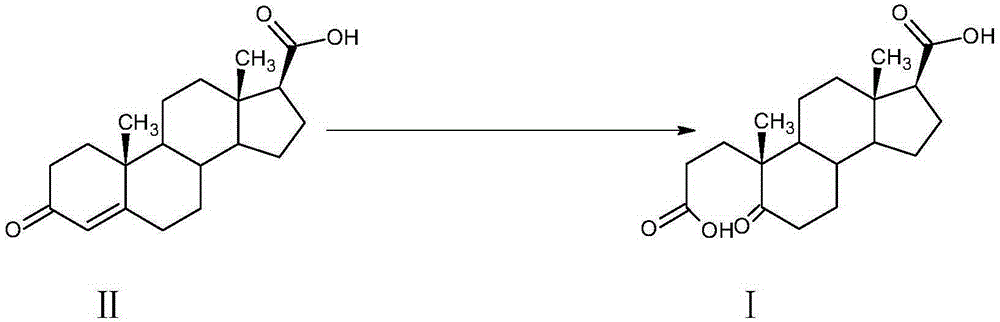
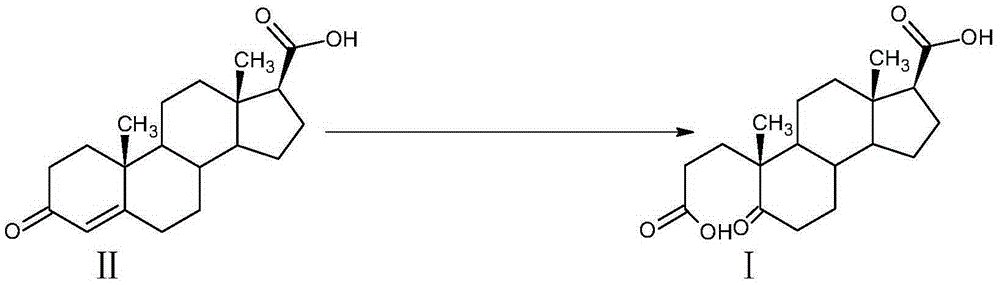
A kind of preparation method of α-abortion-3,5-cracking-androst-5-one-3,17-dioic acid
An androstane and diacid technology is applied in the preparation field of carbocyclic compounds, can solve the problems of high price of iodate, lost products, large solvent consumption and the like, and achieves easy industrial implementation, mild reaction conditions and high raw material recovery rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of A-carbo-3,5-cleavage-androst-5-one-3,17-dioic acid
[0034] At room temperature, in a 3000L enamel reaction kettle equipped with a thermometer, jacket and stirrer, add 1000L of water, stir, add 42kg of sodium carbonate, after dissolving, it becomes alkaline water, add 3-carbonyl-4-androstene-17β - 100kg of carboxylic acid, stirred and dissolved, cooled with circulating water in the jacket.
[0035] Oxidant preparation solution: In a 2000L enamel high-position dripping kettle equipped with a thermometer and a stirrer, add 1500L of water, stir, add 350kg of sodium periodate and 3kg of potassium permanganate, heat to 75-80°C and keep warm to dissolve.
[0036] When the temperature of the circulating water in the reaction kettle is lowered, slowly add the aqueous solution of the oxidant into the reaction kettle dropwise. After the addition is completed within 2 hours, the temperature is controlled at 25°C to 30°C, and the reaction is kept for 2 hou...
Embodiment 2
[0038] Example 2: Preparation of A-carbo-3,5-cleavage-androst-5-one-3,17-dioic acid
[0039] At room temperature, in a 3000L enamel reaction kettle equipped with a thermometer, jacket and stirrer, add 1000L of water, stir, add 53kg of sodium bicarbonate, and dissolve it to obtain alkaline water, add 3-carbonyl-4-androstene- 100kg of 17β-carboxylic acid was stirred and dissolved, and the jacket was cooled by circulating water.
[0040] Oxidant preparation solution: In a 2000L enamel high-position dripping kettle equipped with a thermometer and a stirrer, add 1500L of water, stir, add 350kg of sodium periodate and 3kg of potassium permanganate, heat to 75-80°C and keep warm to dissolve.
[0041] When the temperature of the circulating water in the reaction kettle is lowered, slowly drop the oxidizing agent solution into the reaction kettle. After 1.8 hours of dropping, keep the reaction at 25°C to 30°C for 2 hours, place the plate until the reaction is complete, and then cool do...
Embodiment 3
[0042] Example 3: Preparation of A-carbo-3,5-cleavage-androst-5-one-3,17-dioic acid
[0043] At room temperature, in a 3000L enamel reaction kettle equipped with a thermometer, jacket and stirrer, add 1000L of water, stir, add 16kg of sodium hydroxide, after it dissolves, it becomes alkaline water, add 3-carbonyl-4-androstene- 100kg of 17β-carboxylic acid was stirred and dissolved, and the jacket was cooled by circulating water.
[0044] Oxidant preparation solution: In a 2000L enamel high-position dripping kettle equipped with a thermometer and a stirrer, add 1500L of water, stir, add 350kg of sodium periodate and 3kg of potassium permanganate, heat to 75-80°C and keep warm to dissolve.
[0045] When the temperature of the circulating water in the reaction kettle is lowered, slowly drop the oxidant solution into the reaction kettle. After 1.8 hours of dropwise addition, keep the reaction at 25°C to 30°C for 2 hours, touch the plate until the reaction is complete, cool down to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com