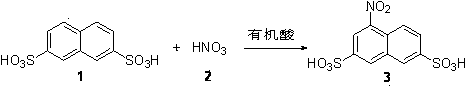
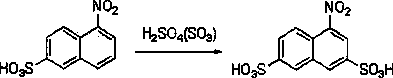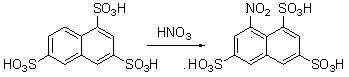Green synthesis method of 4-nitro-2,7-naphthalene disulfonic acid
A naphthalene disulfonic acid, green synthesis technology, applied in the chemical field, can solve problems such as obstacles, and achieve the effects of reducing environmental pollution, overcoming large acid consumption, and improving the cleanliness of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] 0.5 g of 2,7-naphthalene disulfonic acid, 2 g of acetic acid, and 1.2 g of trifluoroacetic acid were added to a 10 ml reaction vessel. The reaction mixture was stirred evenly at 30° C., 0.5 g of nitric acid with a certain mass fraction was added dropwise, and the reaction was tracked by TLC until the raw material was completely converted. After 6 hours the reaction was stopped and the solvent was removed. The crude product was purified by recrystallization to obtain pure 4-nitro-2,7-naphthalene disulfonic acid. After drying, the HPLC detection purity was ≧95%, and the yield was ≧85%. yellow solid 1 H NMR (DMSO- d 6 , 400 MHz): δ = 8.57 (s, 1H, Ar-H), 8.39 (m, 3H, Ar-H), 8.01(d, J = 8.8Hz, 1H, Ar-H); MS (ES-API - ): m / z : 331.9 [(M-H) - ].
example 2
[0030] 0.5 g of 2,7-naphthalene disulfonic acid, 2 g of propionic acid, and 1.6 g of trifluoroacetic acid were added to a 10 ml reaction vessel. The reaction mixture was stirred evenly at 60° C., 0.5 g of nitric acid with a certain mass fraction was added dropwise, and the reaction was tracked by TLC until the raw material was completely converted. After 6 hours the reaction was stopped and the solvent was removed. The crude product was purified by recrystallization to obtain pure 4-nitro-2,7-naphthalene disulfonic acid. After drying, the HPLC detection purity was ≧95%, and the yield was ≧85%. yellow solid 1 H NMR (DMSO- d 6 , 400 MHz): δ = 8.57 (s, 1H, Ar-H), 8.39 (m, 3H, Ar-H), 8.01(d, J = 8.8 Hz, 1H, Ar-H); MS (ES-API - ): m / z expected: 332.9; found: 331.9 [(M-H) - ].
example 3
[0032] 0.5 g of 2,7-naphthalene disulfonic acid, 0.5 g of oxalic acid, and 2 g of acetic acid were added to a 10 ml reaction vessel. The reaction mixture was stirred evenly at 60° C., 0.5 g of nitric acid with a certain mass fraction was added dropwise, and the reaction was tracked by TLC until the raw material was completely converted. After 12 hours the reaction was stopped and the solvent was removed. The crude product was purified by recrystallization to obtain pure 4-nitro-2,7-naphthalene disulfonic acid. After drying, the HPLC detection purity was ≧95%, and the yield was ≧85%. yellow solid 1 H NMR (DMSO- d 6 , 400 MHz): δ = 8.57 (s, 1H, Ar-H), 8.39 (m, 3H, Ar-H), 8.01(d, J = 8.8 Hz, 1H, Ar-H); MS (ES-API - ): m / z : 331.9 [(M-H) - ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com