Combined vaccine for preventing meningitis in children and preparation method of combined vaccine
A combination vaccine and meningitis technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, carrier-bound antigen/hapten components, etc., can solve the problem of no simultaneous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
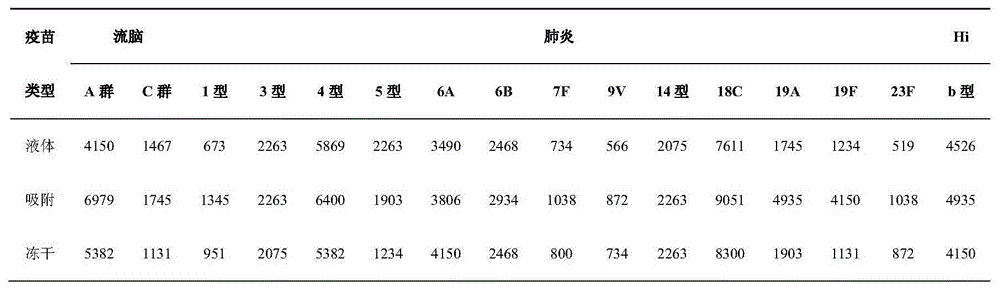
[0060] The preparation of embodiment one capsular polysaccharide
[0061] After bacterial fermentation, collect the supernatant by centrifugation, concentrate by 100Kd ultrafiltration, collect the retentate, add ethanol to 25%, collect the supernatant by centrifugation, add ethanol to 75%, collect the precipitate by centrifugation, and reconstitute with water for injection, hydroxyapatite Chromatography, collect the flow-through, concentrate by 100KD ultrafiltration, clarify by centrifugation, and freeze-dry to prepare capsular polysaccharide.
Embodiment 2
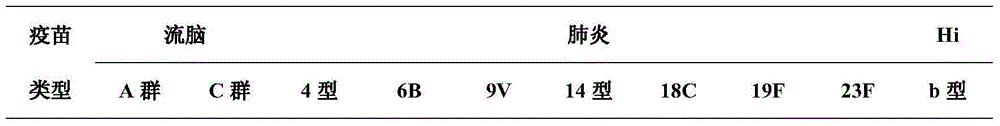
[0062] Embodiment diamine reduction method prepares conjugate (direct connection)
[0063] Polysaccharide dissolved to 5-10mg / ml, add sodium periodate to 2.5-10mmol / L, avoid light oxidation, glycerol terminates the reaction, after 30KD ultrafiltration and concentration, add protein at a ratio of 1:0.5-2, and add borocyanide at the same time Sodium to 2mg / ml, react at room temperature for 3 to 7 days, then purify by molecular sieve chromatography, collect V 0 Nearby elution peaks yield conjugates.
Embodiment 3
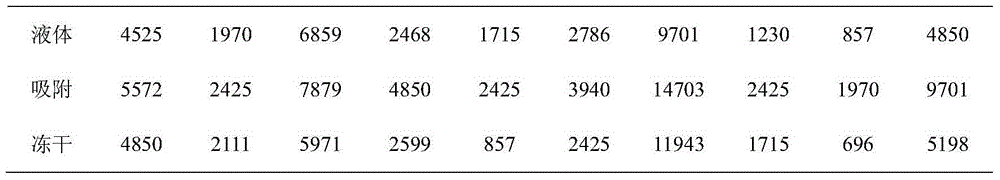
[0064] Embodiment triamine reduction method prepares conjugate (bridging agent connects)
[0065] Polysaccharide dissolved to 5-10mg / ml, add sodium periodate to 2.5-10mmol / L, avoid light oxidation, glycerol terminates the reaction, after 30KD ultrafiltration and concentration, add adipic dihydrazide (ADH) and sodium borocyanide to The final concentration was 40 mg / ml and 2 mg / ml, and reacted at room temperature for 3 days to prepare derivatives.
[0066] Derivatized polysaccharides and carrier proteins are mixed at a mass ratio of 1:0.5~2, carbodiimide (EDAC) is added to a final concentration of 0.02mol / L, and the pH value is maintained at 5.6 for more than 150 minutes. The prepared conjugate is purified by molecular sieve chromatography. collect V 0 Nearby elution peaks were obtained to obtain the stock solution of the conjugate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com