Anti-Nilaparvata-lugens pesticidal protein, and coding gene and application thereof
A coding and protein technology, applied in the field of insecticidal protein against rice brown planthopper and its coding gene and application, can solve the problems of promoting the population of non-target pest insects and the degree of damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1. Acquisition of Novel Insecticidal Protein Gene HY151
[0062] 1. Cry1Ac and Cry54Aa1 protein and gene sequence analysis
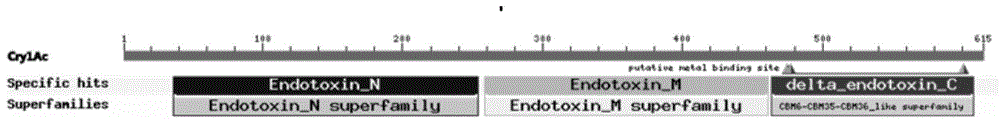
[0063] The amino acid sequences of Cry1Ac and Cry54Aa1 were analyzed using the Blastp function of the NCBI website to analyze their conserved domains, and their corresponding nucleotide sequences were found. It is concluded that the Cry1Ac structural domain I is amino acids 36-254, and the corresponding nucleic acid coding sequence is 106-762 nucleotides; domain II is amino acids 259-461, and the corresponding nucleic acid coding sequence is 775-1383 nucleotides; domain III is amino acids 464-608, the corresponding nucleic acid coding sequence is nucleotides 1390-1824, and the rest of the sequences are linking sequences between domains, such as figure 1 shown. The Cry54Aa1 structural domain I is the 72-316th amino acid, and the corresponding nucleic acid coding sequence is the 214-948th nucleotide; the structural domain II is the 321-514...
Embodiment 2
[0111] Embodiment 2, novel insecticidal protein gene HY151 anti-insect biological activity assay
[0112] The source of tested insects: rice brown planthopper (Nilapavata lugens ) was provided by Lai Fengxiang's laboratory, China Rice Research Institute.
[0113] Samples for testing:
[0114] The anti-BPH protein HY151 prepared in Example 1 is a prokaryotic expression and purified soluble protein. The concentration and yield are shown in the corresponding protein storage tube, sent on dry ice, and stored at -80°C;
[0115] Cry54Aa1 protein: the prokaryotic expression method of Example 1 was used to prepare the Cry54Aa1 protein, and its amino acid sequence was sequence 4 in the sequence listing;
[0116] 54T protein: the prokaryotic expression method of Example 1 was used to prepare 54T protein, and its amino acid sequence was sequence 5 in the sequence listing;
[0117]Cry1Ac protein: using the method of prokaryotic expression in Example 1 to prepare Cry1Ac protein, its am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com